Scientifically Sparing Function Post-Prostatectomy
Spending the past 5 years watching a lot of Disney Junior and reading Dr. Seuss, professional journal reading is generally reserved for the sanctuary of the bathroom. When patients ask if I’ve heard of certain new procedures or therapies, I try to sound intelligent and make a mental note to run a PubMed search on the topic when I get home. Making the effort to stay on top of research, however, makes you a more confident and competent clinician for the information-hungry patient and encourages physicians to respect you when it comes to discussing their patients.
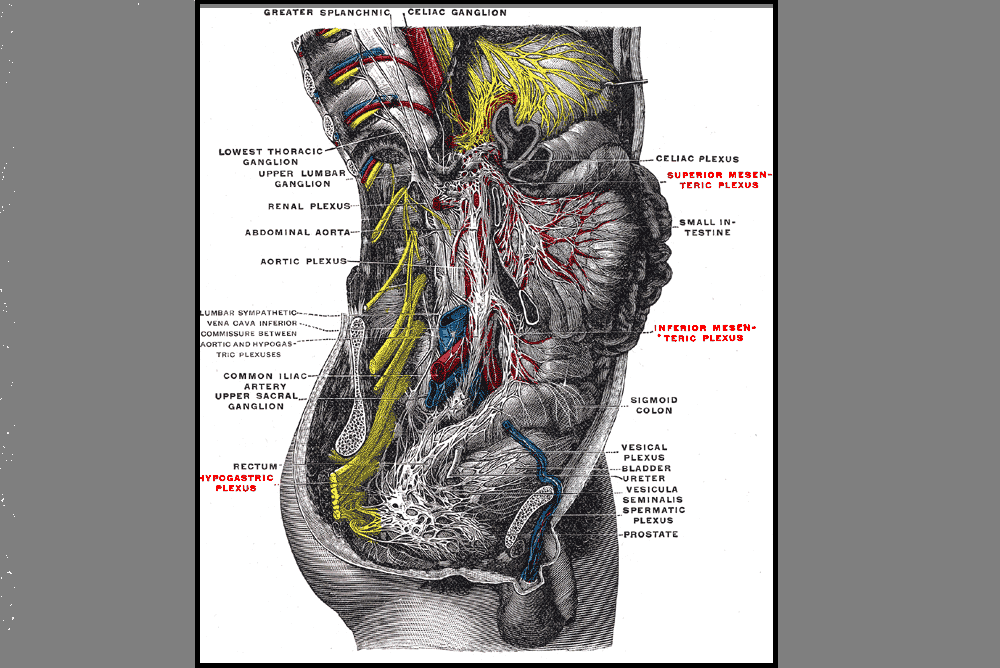
A 2016 article in Translational Andrology and Urology, Lin et al., explored rehabilitation of men post radical prostatectomy on a deeper level, trying to prove that brain-derived neurotrophic factor (BDNF) promotes nerve regeneration. In many radical prostatectomies, even when the nerve-sparing approach is used, there is injury to the cavernous nerves, which course along the posterolateral portion of the prostate. Cavernous nerve injury can cause erectile dysfunction in 60.8-93% of males postoperatively. The authors discussed Schwann cells as being vital for maintaining integrity and function of peripheral nerves like the cavernous nerve. They hypothesized that BDNF, a member of the neurotrophin family that supports neuron survival and prevents neuronal death, activates the JAK/STAT (Janus kinase /signal transducer and activator of transcription) pathway in Schwann cells, thus facilitating axonal regeneration via secretion of cytokines (IL-6 and OSM-M). Through scientific experiment on a cellular level (please refer to the article for the specific details), the authors were able to confirm their hypothesis. Schwann cells do, in fact, produce cytokines that contribute to the regeneration of cavernous nerves.
From a different cellular perspective, Haahr et al., (2016) performed an open-label clinical trial involving intracavernous injection of “autologous adipose-derived regenerative cells” (ADRCs) in males experiencing erectile dysfunction (ED) after radical prostatectomy. Current treatments with PDE-5 inhibitors do not give satisfactory results, so the authors performed a human phase 1, single-arm trial to further the research behind the use of adipose-derived stem cells for ED. Some limitations included the study was un-blinded and had no control group. Seventeen males who had ED after radical prostatectomy 5-18 months prior to the study were followed for 6 months post intracavernosal transplantation. The primary outcome was safety/tolerance of stem cell treatment, and the secondary was improvement of ED. The single intracavernosal injection of freshly isolated autologous adipose-derived cells resulted in 8 of 17 men regaining erectile function for intercourse; however, the men who were not continent did not regain erectile function. The end results showed the procedure was safe and well-tolerated. There was a significant improvement in scores for the International Index of Erectile Function-5 (IIEF-5), suggesting this therapy may be a promising one for ED after radical prostatectomy.
In the clinic, we need to treat our patients to the best of our ability. Taking the Post-Prostatectomy Patient Rehabilitation course is vital if even just one patient enters your office seeking treatment. Keeping up on research (even that which seems too full of forgotten science) and learning new manual techniques and exercises can help us rise as clinicians prepared to optimize patients’ function.
Lin, G., Zhang, H., Sun, F., Lu, Z., Reed-Maldonado, A., Lee, Y.-C., … Lue, T. F. (2016). Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells. Translational Andrology and Urology, 5(2), 167–175. http://doi.org/10.21037/tau.2016.02.03
Haahr, M. K., Jensen, C. H., Toyserkani, N. M., Andersen, D. C., Damkier, P., Sørensen, J. A., … Sheikh, S. P. (2016). Safety and Potential Effect of a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. EBioMedicine, 5, 204–210. http://doi.org/10.1016/j.ebiom.2016.01.024
By accepting you will be accessing a service provided by a third-party external to https://hermanwallace.com./