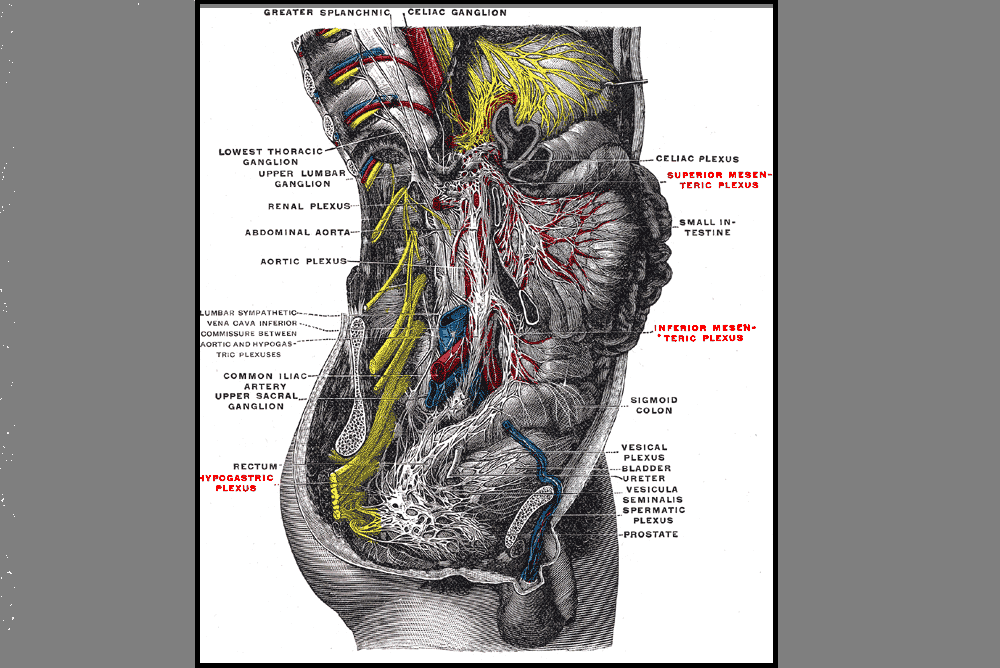
September is Gynae Cancer Awareness Month – but how aware are we as clinicians of the signs and symptoms, the epidemiology and the sequalae of treatment afterwards? As pelvic rehab specialists, we have the privilege of helping women live well after cancer treatment ends, both on a ‘local’ pelvic area (bladder, bowel, sexual and pelvic pain management strategies) but also on a more ‘global’ level – dealing with issues such as cancer related fatigue, bone health and cardiovascular concerns.
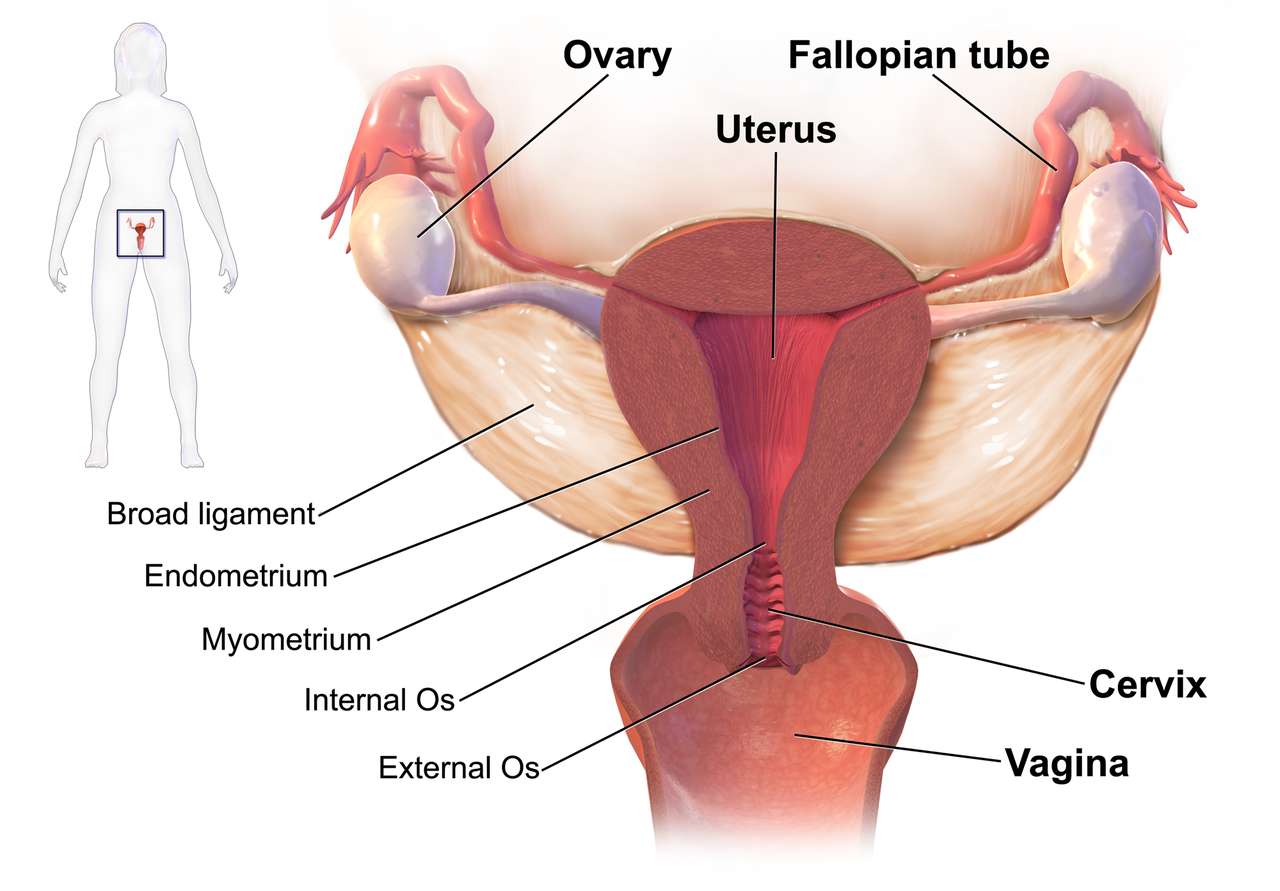 We know that women who are diagnosed with cancer of the vulva, vagina, cervix, endometrium or ovaries are treated with a combination of surgery, radiation or chemotherapy. However, with improving treatment and better survival rates, there is evidence that a variety of pelvic health concerns may arise for these women, both during and after treatment. (Hazewinkel et al 2010). For example, urinary incontinence is reported in 80% of women treated for endometrial cancer, with more severe symptoms and impact on quality of life in those who had adjuvant radiation (Erekson et al 2009) In Malone’s 2017 paper, ‘The patient’s voice: What are the views of women on living with pelvic floor problems following successful treatment for pelvic cancers?’, the author notes that ‘…there is currently a lack of knowledge regarding the effects of PFD on QoL in this cohort. Patients do not always report these problems to their health care providers and clinicians may underestimate symptoms…In the context of having survived cancer, PFD may be seen as relatively trivial. However, in the context of resuming normal living, the symptoms experienced by the survivors may be significant’.
We know that women who are diagnosed with cancer of the vulva, vagina, cervix, endometrium or ovaries are treated with a combination of surgery, radiation or chemotherapy. However, with improving treatment and better survival rates, there is evidence that a variety of pelvic health concerns may arise for these women, both during and after treatment. (Hazewinkel et al 2010). For example, urinary incontinence is reported in 80% of women treated for endometrial cancer, with more severe symptoms and impact on quality of life in those who had adjuvant radiation (Erekson et al 2009) In Malone’s 2017 paper, ‘The patient’s voice: What are the views of women on living with pelvic floor problems following successful treatment for pelvic cancers?’, the author notes that ‘…there is currently a lack of knowledge regarding the effects of PFD on QoL in this cohort. Patients do not always report these problems to their health care providers and clinicians may underestimate symptoms…In the context of having survived cancer, PFD may be seen as relatively trivial. However, in the context of resuming normal living, the symptoms experienced by the survivors may be significant’.
This can present a clinical conundrum – often pelvic rehab therapists are nervous when working with a patient who has a current or previous gynecologic cancer diagnosis, but similarly oncology rehab specialists may have qualms about dealing with pelvic health issues, with the result that these women fall through the cracks and do not have their pelvic health issues managed properly (or at all). Theodore Roosevelt once said ‘No one cares how much you know, until they know how much you care’ and this is especially relevant for oncology pelvic rehab. Often you may be the first clinician to ask about bladder, bowel or sexual function or dysfunction. An understanding of the effects of cancer treatments on the pelvis is important but so too is the wealth of information you may already have about bladder, bowel and sexual health as well as neuroscience and pain education.
The most important thing is to ask these women about their pelvic health concerns – the National Coalition for Cancer Survivorship defined cancer survivorship as extending from ‘the time of diagnosis and for the balance of life’. An emphasis on quality of life has been emphasised – if we know that cancer survivors may not independently volunteer information about their pelvic floor dysfunction, it is our responsibility to ask the questions and comprehensively treat and advocate for these women, in order to help them live well after cancer treatment ends.
- Hazewinkel MH. Sprangers MA, Velden Jvd, Vaart CH, Stalpers LJ, Burger MP ‘Longterm cervical cancer survivors suffer from pelvic floor dysfunction symptoms: A cross-sectional matched cohort study’ Gynecol Oncol 2010;117(2):381-6
- Erekson EA, Sung VW, Disilvestro PA, Myers DL ‘Urinary symptoms and impact on quality of life in women after treatment for endometrial cancer’ Int Urogynecol J 2009;20(2):159-63
- Malone P, Danaher D, Galvin R, Cusack T ‘The patient’s voice: What are the views of women on living with pelvic floor problems following successful treatment for pelvic cancers?’ Physiotherapy Practice and Research 38(2017)93-102
Curing cancer but not addressing life-altering complications can be compared to feeding the homeless on Thanksgiving but turning your back on them the rest of the year. We love hearing positive outcomes of a surgery, but we are not always aware of what happens beyond that. Colorectal cancer is often treated by colectomy, and sometimes the survivor of cancer is left with urological or sexual dysfunction, small bowel obstruction, or pelvic lymphedema.
 Panteleimonitis et al., (2017) recognized the prevalence of urological and sexual dysfunction after rectal cancer surgery and compared robotic versus laparoscopic approaches to see how each impacted urogenital function. In this study, 49 males and 29 females underwent laparoscopic surgery, and 35 males and 13 females underwent robotic surgery. Prior to surgery, 36 men and 9 women were sexually active in the first group and 13 men and 4 women were sexually active in the latter group. Focusing on the male results, male urological function (MUF) scores were worse pre-operatively in the robotic group for frequency, nocturia, and urgency compared to the laparoscopic group. Post-operatively, urological function scores improved in all areas except initiation/straining for the robotic group; however, the MUF median scores declined in the laparoscopic group. Regarding male sexual function (MSF) scores for libido, erection, stiffness for penetration and orgasm/ ejaculation, the mean scores worsened in all areas for the laparoscopic group but showed positive outcomes for the robotic group. In spite of limitations of the study, the authors concluded robotic rectal cancer surgery may afford males and females more promising urological and sexual outcomes as robotic.
Panteleimonitis et al., (2017) recognized the prevalence of urological and sexual dysfunction after rectal cancer surgery and compared robotic versus laparoscopic approaches to see how each impacted urogenital function. In this study, 49 males and 29 females underwent laparoscopic surgery, and 35 males and 13 females underwent robotic surgery. Prior to surgery, 36 men and 9 women were sexually active in the first group and 13 men and 4 women were sexually active in the latter group. Focusing on the male results, male urological function (MUF) scores were worse pre-operatively in the robotic group for frequency, nocturia, and urgency compared to the laparoscopic group. Post-operatively, urological function scores improved in all areas except initiation/straining for the robotic group; however, the MUF median scores declined in the laparoscopic group. Regarding male sexual function (MSF) scores for libido, erection, stiffness for penetration and orgasm/ ejaculation, the mean scores worsened in all areas for the laparoscopic group but showed positive outcomes for the robotic group. In spite of limitations of the study, the authors concluded robotic rectal cancer surgery may afford males and females more promising urological and sexual outcomes as robotic.
Husarić et al., (2016) considered the risk factors for adhesive small bowel obstruction (SBO) after colorectal cancer colectomy, as SBO is a common morbidity that causes a decrease in quality of life. They performed a retrospective study of 248 patients who underwent colon cancer surgery, and 13.7% of all the patients had SBO. Thirty (14%) of the 213 males and 9 (12.7%) of the 71 females had SBO; consequently, they found patients being >60 years old was a more significant risk factor than sex regarding occurrence of SBO. The authors concluded a Tumor-Node Metastasis stage of >3 and immediate postoperative complications were found to be the greatest risk factors for SBO.
Vannelli et al., (2013) explored the prevalence of pelvic lymphedema after lymphadenectomy in patients treated surgically for rectal cancer. Five males and 8 females were examined one week before and 12 months after being discharged from the hospital. All 9 of the patients (4 males, 5 females) with extra-peritoneal cancer exhibited lymphedema via MRI, but the 4 (1 male, 3 females) patients with intra-peritoneal cancer had none. The authors concluded pelvic lymphedema can be elusive after rectal surgery, but pelvic disorders persist and patients should be routinely examined for it.
Obviously saving a life is the primary goal when it comes to cancer. But just like caring for the destitute for one day doesn’t cure a lifetime of hunger, ignoring the negative post-surgical sequelae of a colectomy prevents a cancer survivor from living a healthy life. Herman & Wallace offers two pelvic floor oncology courses, “Oncology and the Male Pelvic Floor” and "Oncology and the Female Pelvic Floor" , which address how pelvic cancers affect the quality of life of our patients and how practitioners can make a positive impact.
Panteleimonitis, S., Ahmed, J., Ramachandra, M., Farooq, M., Harper, M., & Parvaiz, A. (2017). Urogenital function in robotic vs laparoscopic rectal cancer surgery: a comparative study. International Journal of Colorectal Disease, 32(2), 241–248. http://doi.org/10.1007/s00384-016-2682-7
Husarić E., Hasukić Š, Hotić N, Halilbašić A, Husarić S, Hasukić I. (2016). Risk factors for post-colectomy adhesive small bowel obstruction. Acta
 In 1998, faculty member Debora Chassé was asked to evaluate a patient with bilateral lower extremity lymphedema following repeated surgeries for cervical cancer. Her formal education did not cover this in school, so Dr. Chassé began to study peer-review research and consult with other clinicians about the diagnosis. Her journey down the rabbit hole began.
In 1998, faculty member Debora Chassé was asked to evaluate a patient with bilateral lower extremity lymphedema following repeated surgeries for cervical cancer. Her formal education did not cover this in school, so Dr. Chassé began to study peer-review research and consult with other clinicians about the diagnosis. Her journey down the rabbit hole began.
Dr. Chassé became a certified lymphedema therapist in 2000 and a certified Lymphology Association of North America therapist in 2001. She continued training by moving into osteopathy taking her into the direction of lymphatic vessel manipulation. In 2006 she began taking courses in pelvic pain and obstetrics with a focus on pelvic floor dysfunction. It was at this point that Dr. Chasse realized nobody was applying lymphatic treatment to women’s health and pelvic floor dysfunction. In 2009 she became a Board Certified Women’s Health Clinical Specialist in Physical Therapy and began traveling around the United States offering workshops in the area of lymphatic treatment.
Dr. Chassé’s approach is to incorporate all her varied skills in the clinic to produce the best patient outcomes. Debora explains that she is “…showing the similarities between pelvic pain and the lymphatic system. The treatment principles are the same, when you are treating both lymphedema or pelvic pain, you are working to reduce inflammation, pain and scarring.”
Another advantage of the lymphatic treatment approach is that it is more comfortable for the patient. “Most intravaginal techniques causes increased pain and inflammation. However, using lymphatic drainage intravaginally is well tolerated and decreases the intravaginal pain. The results are phenomenal!”
Dr. Chassé recollects her experience with a 21 year old female who suffered from chronic pelvic pain. By applying intravaginal lymphatic drainage techniques for 5 consecutive days, the patient experience a 4.83 reduction in pelvic girdle circumference and her intravaginal pain went from 8/10 to 2/10. The patient was amazed at how much better she felt. “My pants fit better, my energy level increased 25% and pain decreased more than 50%. I went from having 2-3 bad days per week to having 2-3 bad days per month, even when my work level increased. My feet no longer swell and I haven’t missed any classes since receiving this treatment.
In her course, “Lymphatics and Pelvic Pain: New Strategies”, Dr. Chassé seeks to train practitioners to utilize lymphatic drainage techniques when treating specifically pelvic pain. Participants will learn lymphatic drainage principles and techniques. They will learn how to clear pathways to transport lymph fluid and internal techniques which will have incredible impacts for patients.
Lymphedema with regards to women’s health is most commonly associated with breast cancer. Upper extremity lymphedema can be limiting and painful without a doubt, and I have seen women suffering from irritating edema and limited shoulder range of motion and function after radical mastectomy. However, we may not always consider lower extremity lymphedema which can occur as a result of urogenital cancers and their treatments. Our knowledge and skilled hands can impact the quality of life of these patients who may seek treatment for their post-cancer complications.
 Mitra et al., published a 2016 retrospective study on lymphedema risk post radiation therapy in endometrial cancer. They considered 212 endometrial cancer survivors, and 7.1% who received adjuvant pelvic radiation therapy developed lower extremity lymphedema after treatment, whether they had chemotherapy or not. Finding at least 1 positive pathological lymph node was directly correlated with an increased risk of lymphedema, regardless of attempts to control pelvic lymph-node dissection. These statistics encourage finding prophylactic measures to take for stage III endometrial cancer patients to minimize the risk for long-term lymphedema. Regarding treatment for lower extremity lymphedema, this paper discussed compression stocking use, pneumatic compression stockings, and complex decongestive therapy, an intensive regimen of physical therapy and massage that is unfortunately not easily accessible for a majority of patients. The authors encouraged future research on the efficacy of exercise and compression for lymph node positive patients.
Mitra et al., published a 2016 retrospective study on lymphedema risk post radiation therapy in endometrial cancer. They considered 212 endometrial cancer survivors, and 7.1% who received adjuvant pelvic radiation therapy developed lower extremity lymphedema after treatment, whether they had chemotherapy or not. Finding at least 1 positive pathological lymph node was directly correlated with an increased risk of lymphedema, regardless of attempts to control pelvic lymph-node dissection. These statistics encourage finding prophylactic measures to take for stage III endometrial cancer patients to minimize the risk for long-term lymphedema. Regarding treatment for lower extremity lymphedema, this paper discussed compression stocking use, pneumatic compression stockings, and complex decongestive therapy, an intensive regimen of physical therapy and massage that is unfortunately not easily accessible for a majority of patients. The authors encouraged future research on the efficacy of exercise and compression for lymph node positive patients.
Shaitelman et al. presented a review of the progress made in the treatment and prevention of cancer-related lymphedema (2015). They stated gynecologic cancer treatment is associated with 25% incidence of lymphedema. Endometrial cancer had 1%, cervical cancer had 27%, and vulvar cancer had 30% incidence specifically. Sentinel lymph node biopsy (SLNB) can be an important part of cancer treatment, as lymphedema incidence was shown to average 9%. With treatment of genitourinary cancers, lymphedema occurred in 4% patients with prostate cancer, 16% patients with bladder cancer, and 21% patients with penile cancer. Shown to decrease limb volume and improve quality of life, the current standard of care is complete decongestive therapy (CDT). These authors state CDT involves the use of manual lymphatic drainage (MLD), bandaging on a daily basis, skin care, exercise, and a 3-phase protocol of compression. The use of SLNB helps identify the risk of lymphedema post cancer treatment; however, clinicians need to be aware of the signs and symptoms of lymphedema so the affected patient can be recognized early and referred to the appropriate specialist for treatment.
We may be referred patients with the confirmed diagnosis of lymphedema, but often we see post-cancer patients for rehab who develop lymphedema months after radiation or chemotherapy. Any healthcare professional involved with regular treatment of a cancer-surviving patient needs to have a keen sense for diagnosing and properly taking care of lymphedema. Courses such as “Lymphatics and Pelvic Pain: New Strategies” can open a whole new avenue for understanding what patients may need from us following urogenital and genitourinary cancers. Why not be prepared to face with knowledge and skill whatever pathology our patients present?
Mitra, D., Catalano, P.J., Cimbak, N., Damato, A.L., Muto, M.G., & Viswanathan, A.N. (2016). The Risk of Lymphedema After Postoperative Radiation Therapy in Endometrial Cancer. Journal of Gynecologic Oncology, 27(1), e4.
Shaitelman, S.F., Cromwell, K.D., Rasmussen, J.C., Stout, N.L., Armer, J.M., Lasinski, B.B., & Cormier, J.N. (2015). Recent Progress in Cancer-Related Lymphedema Treatment and Prevention. CA: A Cancer Journal for Clinicians, 65(1), 55-81.