In an effort to provide the best possible educational experience for clinical rehabilitation application of neuroanatomy, I was on a mission. Having a core, base knowledge review of the nervous system is essential when leading into talking about dysfunction and disease of that system. I went on a search for anatomical depictions that could clearly identify the structures and processes I was trying to portray. New books from the library and books I own from when I was in college serve as great resources when trying to get back into studying the specifics, but do not offer the opportunity to easily get these images into a powerpoint. Online resources are also challenging. I am learning how time consuming the process can be to determine who owns the online image, if it is free to copy, save and utilize for my own teaching purposes, or if I need to go through the process of requesting permissions for use.
Through my employer, where I treat patients in the clinic, I have access to a program called Primal Pictures. I had used this in the past for clinic related marketing presentations and educational materials for patients and other clinicians I have mentored. Looking into the product further, I came to find out that there is a newer version of the program which offered so many more options. A truly unlimited amount of images which can be manipulated into an optimal position depicting the most clear neuroanatomical views I have ever been able to find. Not only does it provide me with the images I need in order to depict the treacherous pathways of the nerves in our body, but it also provides some amazing depictions of the physiological processes that occur within our nervous system to allow for healthy day to day functioning and protection of our bodies.
I also came across the title of a journal article that I was sure would provide some excellent depictions of neuroanatomy. The article titled, Sectional Neuroanatomy of the Pelvic Floor, provides cross sectional views of both the male and female pelvises. I obtained the article which has an excellent color-coded system, each nerve colored the same as the muscles and skin surface it innervates, going from superior to inferior cross sections. This makes for a clear understanding of each structures anatomical position. It is a great reference when looking at the anatomical relationships to adjacent structures and can help guide palpation skills. The article was more specifically written for physicians to best direct needle procedures/injections in the most accurate location possible when targeting nerves and structures. Neuroanatomy and physiology can be essential to understanding certain patient populations we encounter as we practice pelvic floor rehabilitation. Having clear depictions to refer to can help you provide the best possible base knowledge to your patients as you help them understand the challenges they face and how to overcome them.
Kass, J. S., Chiou-Tan, F. Y., Harrell, J. S., Zhang, H., & Taber, K. H. (2010). Sectional neuroanatomy of the pelvic floor. Journal of computer assisted tomography, 34(3), 473-477.
In 2007, after only speaking on the phone and never meeting in person, my new friend and colleague Stacey Futterman and I presented at the APTA National Conference on the topic of male pelvic pain. It was a 3 hour lecture that Stacey had been asked to give, and she invited me to assist her upon recommendation of one of her dear friends who had heard me lecture. I still recall the frequent glances I made to match the person behind the voice I had heard for so many long phone calls.
 Upon recommendation of Holly Herman, we took this presentation and developed it into a 2 day continuing education course, creating lectures in male anatomy (we definitely did not learn about the epididymis in my graduate training), post-prostatectomy urinary incontinence, pelvic pain, and a bit about sexual health and dysfunction. Although it truly seems like the worst imaginable question, we asked each other “should we allow men to attend?” As strange as this question now seems, it speaks volumes about the world of pelvic health at that time; mostly female instructors taught mostly female participants about mostly female conditions.
Upon recommendation of Holly Herman, we took this presentation and developed it into a 2 day continuing education course, creating lectures in male anatomy (we definitely did not learn about the epididymis in my graduate training), post-prostatectomy urinary incontinence, pelvic pain, and a bit about sexual health and dysfunction. Although it truly seems like the worst imaginable question, we asked each other “should we allow men to attend?” As strange as this question now seems, it speaks volumes about the world of pelvic health at that time; mostly female instructors taught mostly female participants about mostly female conditions.
Make no mistake- women’s health topics were and are deserving of much attention in our typically male-centered world of medicine and research. Maternal health in the US is dreadful, and gone are the days when providers should allow urinary incontinence or painful sexual health to be “normal”, yet it is often described as such to women who are brave enough to ask for help. Times have changed for the better for us all.
The Male Pelvic Floor Course was first taught in 2008, and so far, 22 events have taken place in 18 different cities. 73 men have attended the course to date, with increasing numbers represented at each course. Rather than 20-25 attendees, the Institute is seeing more of the men’s health course filling up with 35-40 participants. In my observations, the men who attend the course are often very experienced, have excellent orthopedic and manual therapy skills, and have personalities that fit very well into the sensitive work that is pelvic rehabilitation.
The course was expanded to include 3 days of lectures and labs, and this expansion allowed more time for hands-on skills in examination and treatment. The schedule still covers bladder, prostate, sexual health and pelvic pain, and further discusses special topics like post-vasectomy syndrome, circumcision, and Peyronie’s disease. In my own clinical practice, learning to address penile injuries has allowed me to provide healing for conditions that are yet to appear in our journals and textbooks. As I often say in the course, we are creating male pelvic rehabilitation in real time.
Because the course often has providers in attendance who have not completed prior pelvic health training, instruction in basic techniques are included. For the experienced therapists, there are multiple lab “tracks” that offer intermediate to advanced skills that can be practiced in addition to the basic skills. Adaptations and models are used when needed to allow for draping, palpation, and education when working with partners in lab, and space is created for those therapists who want to learn genital palpation more thoroughly versus those who are deciding where their comfort zone is at the time. One of the more valuable conversations that we have in the course is how to create comfort and ease in when for most us, we were raised in a culture (and medical training) where palpation of the pelvis was not made comfortable. Hearing from the male participants about their bodies, how they are affected by cultural expectations, adds significant value as well.
We need to continue to create more coursework, more clinical training opportunities so that the representation of those treating male patients improves. If you feel ready to take your training to the next level in caring for male pelvic dysfunction, this year there are three opportunities to study. I hope you will join me in Male Pelvic Floor Function, Dysfunction and Treatment.
I love adding flax seed to my recipes when I bake. I even hide it in yogurt with crushed graham crackers for my kids. It is a powerful nutrient that can be consumed without knowing it! Although the specific mechanism for its efficacy on prostate health continues to be researched, studies over the last several years applaud flax seed for its benefits and encourage me to keep sneaking it in my family’s diet.
 In 2008, Denmark-Wahnefried et al. performed a study to see if flax seed supplementation alone (rather than in combination with restricting dietary fat) could decrease the proliferation rate of prostate cancer prior to surgery. Basically, flax seed is a potent source of lignan, which is a phytoestrogen that acts like an antioxidant and can reduce testosterone and its conversion to dihydrotestosterone. It is also rich in plant-based omega-3 fatty acids. In this study, 161 prostate cancer patients, at least 3 weeks prior to prostatectomy, were divided into 4 groups: 1) normal diet (control); 2) 30g/day of flax seed supplementation; 3) low-fat diet; and 4) flax seed supplementation combined with low-fat diet. Results showed the rate of tumor proliferation was significantly lower in the flax seed supplemented group. The low-fat diet was proven to reduce serum lipids, consistent with previous research for cardiovascular health. The authors concluded, considering limitations in their study, flax seed is at least safe and cost-effective and warrants further research on its protective role in prostate cancer.
In 2008, Denmark-Wahnefried et al. performed a study to see if flax seed supplementation alone (rather than in combination with restricting dietary fat) could decrease the proliferation rate of prostate cancer prior to surgery. Basically, flax seed is a potent source of lignan, which is a phytoestrogen that acts like an antioxidant and can reduce testosterone and its conversion to dihydrotestosterone. It is also rich in plant-based omega-3 fatty acids. In this study, 161 prostate cancer patients, at least 3 weeks prior to prostatectomy, were divided into 4 groups: 1) normal diet (control); 2) 30g/day of flax seed supplementation; 3) low-fat diet; and 4) flax seed supplementation combined with low-fat diet. Results showed the rate of tumor proliferation was significantly lower in the flax seed supplemented group. The low-fat diet was proven to reduce serum lipids, consistent with previous research for cardiovascular health. The authors concluded, considering limitations in their study, flax seed is at least safe and cost-effective and warrants further research on its protective role in prostate cancer.
In 2017, de Amorim et al. investigated the effect of flax seed on epithelial proliferation in rats with induced benign prostatic hyperplasia (BPH). The 4 experimental groups consisting of 10 Wistar (outbred albino rats) rats each were as follows: 1) control group of healthy rats fed a casein-based diet (protein in milk); 2) healthy rats fed a flax seed-based diet; 3) hyperplasia-induced rats fed a casein diet; and 4) hyperplasia-induced rats fed a flax seed diet. Silicone pellets full of testosterone propionate were implanted subcutaneously in the rats to induce hyperplasia. Once euthanized at 20 weeks, the prostate tissue was examined for thickness and area of epithelium, individual luminal area, and total prostatic alveoli area. Results showed the hyperplasia induced rats fed a flax seed-based diet had smaller epithelial thickness as well as a reduced proportion of papillary projections found in the prostatic alveoli. These authors determined flax seed exhibits a protective role for the epithelium of the prostate in animals induced with BPH.
Bisson, Hidalgo, Simons, and Verbruggen2014 hypothesized a lignan-fortified diet could decrease the risk of BPH. The authors used an extract rich in lignan obtained from flax seed hulls. Four groups of 12 Wistar rats were used, with 1 negative control group and 3 groups with testosterone propionate (TP)-induced BPH (1 positive control, and 2 with diets containing 0.5% or 1.0% of the extract). Over a 5 week period, the 2 BPH-induced groups consuming the lignan extract starting 2 weeks prior to the BPH induction demonstrated a significant inhibition of prostate growth from the TP compared to the positive control group. These authors concluded the lignan-rich flax seed hull extract prevented BPH induction.
From BPH to prostate cancer, flax seed has proven a noteworthy supplement for preventative health. A tablespoon of flax seed in a muffin recipe is likely not a life-changing dose, but it’s a start. Nutrition Perspectives for the Pelvic Rehab Therapist enlightens practitioners with even more healthy choices, and Post-Prostatectomy Patient Rehabilitation gives you the necessary tools to help patients recover from prostate cancer.
Demark-Wahnefried, W., Polascik, T. J., George, S. L., Switzer, B. R., Madden, J. F., Ruffin, M. T., … Vollmer, R. T. (2008). Flax seed Supplementation (not Dietary Fat Restriction) Reduces Prostate Cancer Proliferation Rates in Men Presurgery. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 17(12), 3577–3587. http://doi.org/10.1158/1055-9965.EPI-08-0008
de Amorim Ribeiro, I.C., da Costa, C.A.S., da Silva, V.A.P. et al. (2017). Flax seed reduces epithelial proliferation but does not affect basal cells in induced benign prostatic hyperplasia in rats. European Journal of Nutrition. 56: 1201. https://doi.org/10.1007/s00394-016-1169-1
Bisson JF, Hidalgo S, Simons R, Verbruggen M. 2014. Preventive effects of lignan extract from flax hulls on experimentally induced benign prostate hyperplasia. Journal of Medicinal Food. 17(6): 650-656. http://doi.org/10.1089/jmf.2013.0046
The following is the first in a series of posts by Erica Vitek, MOT, OTR, BCB-PMD, PRPC. Erica joined the Herman & Wallace faculty in 2018 and is the author of Neurologic Conditions and Pelvic Floor Rehab.

Looking back, being passionate about how to physically exercise a person with Parkinson disease to produce the best functional outcome actually became a passion of mine when I was offered my first job. I was thrown into treating people with Parkinson disease in an acute care setting. I had very limited knowledge about Parkinson disease at the time, but I learned quickly from the vast opportunity that was offered to me through my place of work, which was the regions sought after Parkinson disease center of excellence. At the same time, I was eager to further advance my skills as a pelvic floor therapist, which I developed a substantial interest in when I was in college.
As I learned more about what people with Parkinson disease had to manage in their daily lives, it became very clear to me that autonomic dysfunction was a very challenging, and sometimes disabling, aspect of the disease. Being knowledgeable about the neurological and musculoskeletal system along with the urinary, gastrointestinal, and sexual systems seemed to fit well together but there was no specific place to go to combine this knowledge. The research I began collecting on this topic was abundant and very intriguing. Bringing this information together could be practice changing for me to help people living with Parkinson disease.
As clinicians, we already know how to be understanding about the very personal details of the people we work with. People with Parkinson disease deal with an extra layer of challenge, such as, bradykinesia, freezing of gait, and tremor affecting their day to day self-care and relationships. Adding urinary incontinence, constipation or sexual dysfunction to the list makes for even more difficult management.
How does one clinician share their passion with other clinicians that also have the same desires to give the best care to their patients with Parkinson disease? Having a great deal of respect for Herman and Wallace and what they have to offer clinicians practicing pelvic rehabilitation, it seemed like it could be the perfect fit for a course like this. The work that would lie ahead if this idea took off was overwhelming but did not hinder me from my proposal. In fact, it has led to an even larger scope addressing the of treatment of the pelvic floor for a multitude of neurologic conditions many of us see daily in our clinics. Pulling it all together to share is a process that will reward not only people with Parkinson disease in my practice but hopefully yours as well.
Recently in the Pelvic Rehab Report blog we discussed the beneficial role of pelvic rehabilitation for symptoms of dysmenorrhea. Additional research was published this year that supports the use of pranayama for improving quality of life and pain in girls with primary dysmenorrhea. Breathing within yoga studies is a rich field, with well-defined variations in stages and kinds of breathing, techniques and postures, and use of different hand positions and breathing through the nostrils and/or mouth. The Oxford online dictionary defines pranayama as a practice coming from Hindu yoga and related to regulating the breath through specific techniques.

In the study, the practice of both slow pranayama (Nadi Shodhan) and fast pranayama (Kapalbhati) was instructed to the women to be completed in the mornings on an empty stomach for 10 minutes per day. Ninety unmarried young women (ages 18-25) diagnosed with primary dysmenorrhea were randomly and equally assigned to either Group A (slow pranayama) or Group B (fast pranayama). Outcomes included the Moos menstrual distress questionnaire (MMDQ), numerical pain rating pain scale, a quality of life scale "by American chronic pain association" and the assessments were administered at baseline, after the first menstrual cycle, and after the second menstrual cycle. To read more details about the methods and results, the full article can be accessed here.
Prior and recent research has also studied the effects of similar breathing techniques on cognitive functions in healthy adults and also on perceived stress and cardiovascular parameters in young healthcare students. While it may not be new to compare fast and slow pranayama techniques with health conditions, this is the first study to address pranayama's effects on symptoms of dysmenorrhea. The authors conclude that practicing slow pranayama compared to fast pranayama improved quality of life and pain scores related to dysmenorrhea. Furthermore, the authors suggest that because pranayama can decrease absenteeism and stress levels, the practice should be implemented in college students to improve quality of life.
If you are looking to learn more about pranayama and other methods of self-management of conditions including, but certainly not limited to, dysmenorrhea, come to the city-New York City- next month for Meditation for Patients and Providers instructed by faculty member Nari Clemons. It's sure to be hot in the city, so chill out indoors with Nari, and hang out at night with your new favorite colleagues that you'll meet. A benefit of this course is that not only can you learn to care better for your patients, but also for yourselves, and you deserve it.
The expression, “the canary in the coal mine” comes from a long ago practice of coalminers bringing canaries with them into the coalmines. These birds were more sensitive than humans to toxic gasses and so, if they became ill or died, the coalminers knew they had to get out quickly. The canaries were a kind of early warning signal before it was too late. Even though the practice has been discontinued, the metaphor lives on as a warning of serious danger to come.
Osteoporosis, which means porous bones, has been called a silent disease because often an individual doesn’t know he or she has it until they break a bone. The three common areas of fracture are the wrist, the hip, or the spine. Osteoporosis fractures are called fragility fractures, meaning they happen from a fall of standing height or less. We should not break a bone just by a fall unless there is an underlying cause which makes our bones fragile.
 Wrist fractures typically happen when a person starts to fall and puts his or her arms out to catch themselves. They often are seen in the Emergency Department but seldom followed up with an Osteoporosis workup. According to the International Osteoporosis Foundation’s Capture the Fracture program, 80% of fracture patients are never offered screening and / or treatment for osteoporosis. As professionals working with patients who often have co-morbidities, we can be the ones to screen for osteoporosis and balance problems, particularly if our patients have a history of fractures. These screens include the following:
Wrist fractures typically happen when a person starts to fall and puts his or her arms out to catch themselves. They often are seen in the Emergency Department but seldom followed up with an Osteoporosis workup. According to the International Osteoporosis Foundation’s Capture the Fracture program, 80% of fracture patients are never offered screening and / or treatment for osteoporosis. As professionals working with patients who often have co-morbidities, we can be the ones to screen for osteoporosis and balance problems, particularly if our patients have a history of fractures. These screens include the following:
1. Check for the three most common signs of osteoporosis:
a. History of fractures
b. Hyper-kyphosis of the thoracic spine
c. Loss of height equal or greater than 4 cm.
2. Grip Strength
Low grip strength in women is associated with low bone density1
3. Rib-pelvic distance- less than two fingerbreadths.
With the patient standing with their back to you, arms raised to 90 degrees, check the distance from the lowest rib to the iliac crest. Two fingerbreadths or less may be indicative of a vertebral fracture.
A prior fracture is associated with an 86% increased risk of any fracture based on a 2004 meta-analysis by Kanis, Johnell, and De Laet in Bone 2. Fracture predicts fracture. It is our duty as professionals and as human beings to intervene by screening and referring out even if this is not the primary reason we are treating this patient. Fractures from osteoporosis can be devastating, resulting in increased risk of mortality at worst and a diminished quality of life at best. Look for the canaries in the coal mine. Our patients deserve to live the quality of life they envision.
Deb Gulbrandson, PT, DPT, CEEAA teaches the Meeks Method for Osteoporosis Management seminars for Herman and Wallace around the country.
1. Dixon WG et al. Low grip strength is associated with bone mineral density and vertebral fracture in women. Rheumatology 2005;44:642-646
2. Kanis JA, Johnell O, De Laet C, et al. (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375
Influencing pelvic floor EMG activity through hip joint mobilization and positioning
EMG is a helpful tool to observe pelvic floor muscle activity and how it is influenced by everything from regional musculoskeletal factors and mucosal health, to client motor control, awareness, and comfort.
In this post I will discuss the case of one client who was referred for dyspareunia treatment, and whose SEMG findings are outlined in Figures 1-3. She had validated test item clusters for right hip labral tear as well as femoral acetabular impingement, in addition to right sided pelvic floor muscle overactivity and sensitivity with less than 3 ounces of palpation pressure.
The figures below demonstrate peri-anal SEMG response of pelvic floor muscles within a single treatment session, which included sacral unloading in supine as well as hip joint mobilization to demonstrate the relationship between her pelvic floor and her hips. Our focus for this SEMG downtraining treatment was to enable her to understand the connection between her pelvic floor muscle holding patterns and her body’s preferences to remain out of ranges of motion that impinged and irritated her hip.
By creating a clear understanding of how the client could 'listen" to her muscle activity via SEMG (as well as her kinesthetic awareness of her own comfort), she began to understand the difference between body and hip position, her pelvic floor muscle activity, and her pain during intercourse.
Pelvic floor motor control with normalized respiration, orthopedic considerations of sexual activity, and other physical therapy as well as multidisciplinary treatments were integrated into her ability to resume intercourse. The lens of SEMG, however, was a powerful tool to help her make the connection between her hip and its influence on her pelvic floor overactivity and symptoms.
Musculoskeletal co-morbidities in pelvic pain are common, requiring the clinician to have a set of test item clusters to scan and clear key structures, as well as the ability to convey this information without creating distress to the client when positive findings are discovered. For example, labral tears and subchondral cysts are common findings in asymptomatic clients and physical therapy plays a key role in reducing client fear, avoiding symptom provocation, reducing regional muscle overactivity, as well as facilitating movement and strengthening in painfree ROM.
Although this case example describes intraarticular hip dysfunction as a driver of this clients PFM overactivity, Finding the Driver in Pelvic Pain is a course that is designed to cover comprehensive key test item clusters for a fundamental pelvic pain scan exam of intrapelvic as well as extrapelvic drivers, to ensure the clinician understands the contributing factors that can influence or be influenced by the pelvic floor. This course is best suited for physical therapists and physical therapist assistants who are looking to create an organized approach to their scan exam for pelvic pain. For non-physical therapists, this can be a powerful introduction to the skill set and vocabulary needed to create a multidisciplinary team with a PT in the treatment of these clients.
FIGURE 1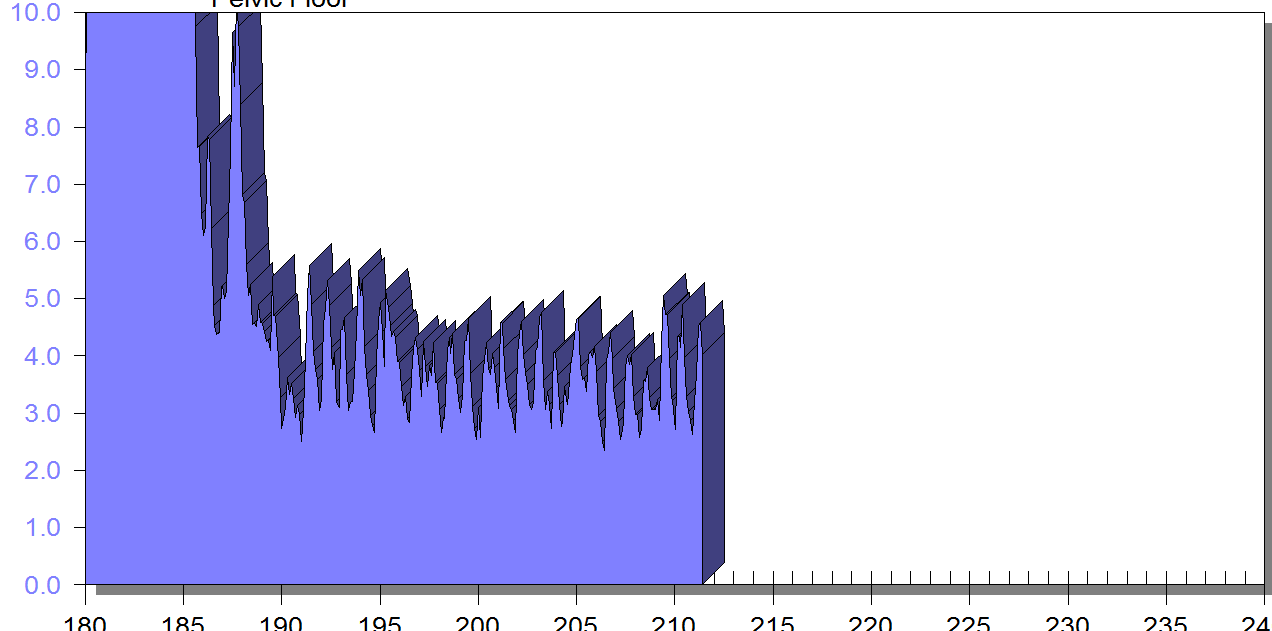
PFM EMG at rest in supine, knees bent, feet on table (peri-anal SEMG electrode placement)
FIGURE 2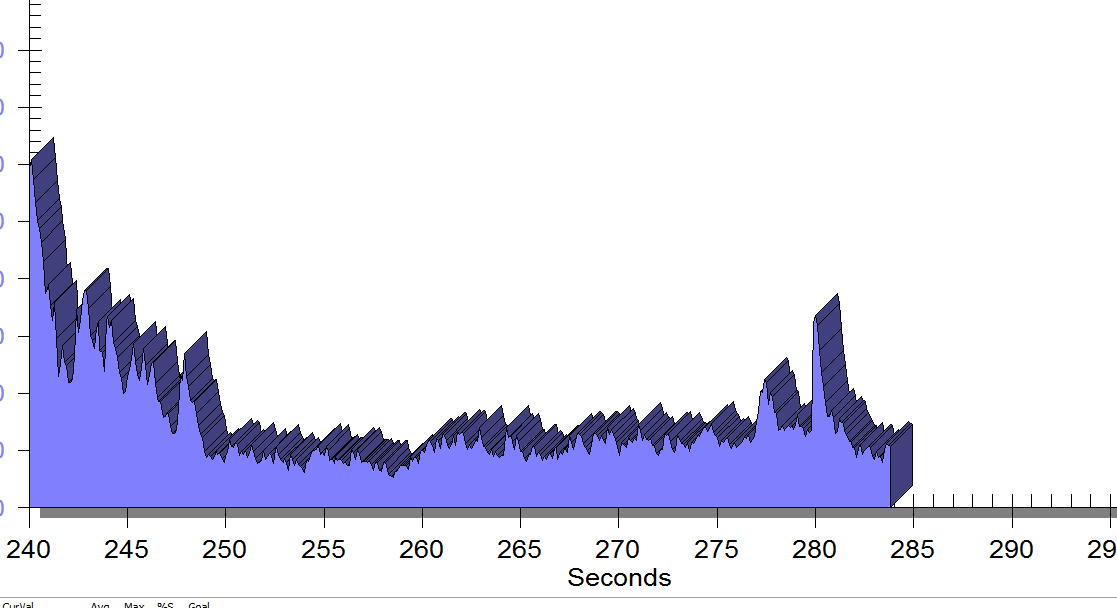
Same position, only with sacral unweighting by placing folded towels on either side of sacrum, unweighting all pressure from sacrum. Immediate report of increased comfort in buttocks, hips and pelvis.
Figure 3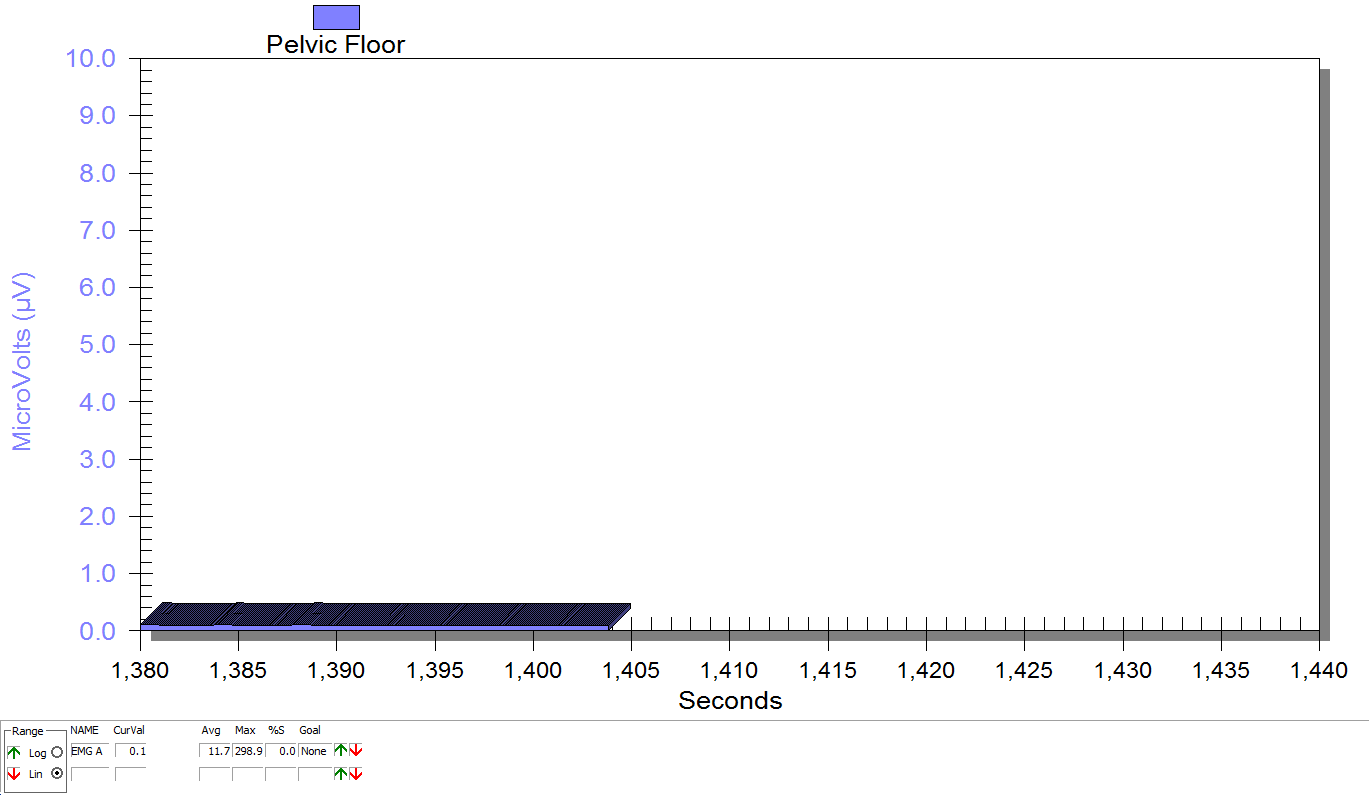
Supine, sacrum unweighted as in figure 2, after multidirecitonal hip joint mobilizaiton.
Groh, Herrera. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009 Jun; 2(2): 105–117. Published online 2009 Apr 7. doi: 10.1007/s12178-009-9052-9
Yosef, et al. Multifactorial contributors to the severity of chronic pelvic pain in women. Am J Obstet Gynecol. 2016 Dec;215(6):760.e1-760.e14. doi: 10.1016/j.ajog.2016.07.023. Epub 2016 Jul 18.
It’s St Valentine’s day this week – you may have noticed hearts and flowers everywhere you look and a general theme of love and romance. For many women going through cancer treatment, sex may be the last thing on their mind…or not! Women who are going through treatment for gynecologic cancer are often handed a set of dilators with minimal instruction on how to use them, or as one patient reported, they are told to have sex three or four times a week during radiation therapy ‘to keep your vagina patent’. As a pelvic rehab practitioner with a special interest in oncology rehab, I know that we can (we must!) do better, in helping women live well after cancer treatment ends.
 As Susan Gubar, an ovarian cancer survivor, writes in the New York Times ‘…It can be difficult to experience desire if you don’t love but fear your body or if you cannot recognize it as your own. Surgical scars, lost body parts and hair, chemically induced fatigue, radiological burns, nausea, hormone-blocking medications, numbness from neuropathies, weight gain or loss, and anxiety hardly function as aphrodisiacs…’
As Susan Gubar, an ovarian cancer survivor, writes in the New York Times ‘…It can be difficult to experience desire if you don’t love but fear your body or if you cannot recognize it as your own. Surgical scars, lost body parts and hair, chemically induced fatigue, radiological burns, nausea, hormone-blocking medications, numbness from neuropathies, weight gain or loss, and anxiety hardly function as aphrodisiacs…’
Although sexual changes can be categorised into physical, psychological and social, the categories cannot be neatly delineated in the lived experience (Malone at al 2017). The good news? Pelvic rehab therapists not only have the skills to enhance pelvic health after cancer treatment and are ideally positioned to be able to take a global and local approach to the sexual health difficulties women may face after cancer treatment ends, but there is also a good and growing body of evidence to support the work we do. Factors to consider include physical issues leading to dyspareunia, including musculo-skeletal/ orthopaedic, Psychological issues, including loss of libido and other pelvic health issues impacting sexual function such as faecal/ urinary incontinence, pain or fatigue.
In Hazewinkel’s 2010 paper, women reported that they thought their physicians would tell them if solutions were available…most reported reasons for not seeking help were that women found their symptoms bearable in the light of their cancer diagnosis and lacked knowledge about possible treatments but when informed of possible treatment strategies ‘…women stated that care should be improved, specifically by timely referral to pelvic floor specialists’. The good news: ‘‘Pelvic Floor Rehab Physiotherapy is effective even in gynecological cancer survivors who need it most.’ (Yang 2012)
The issue therefore may be one of awareness – for both the women who need our services and the physicians and healthcare team who work in the field of gynecologic oncology. What we need is acknowledgement of the issues and confident conversation and assessment by clinicians – interested in learning more? Come and join the conversation in Tampa next month at my Oncology & the Female Pelvic Floor course!
‘Sex after Cancer’ by Susan Gubar, https://www.nytimes.com/2018/01/18/well/live/sex-after-cancer.html
Malone et al 2017: ‘‘The patient’s voice: What are the views of women on living with pelvic floor problems following successful treatment for pelvic cancers?’
Hazewinkel et al 2010 ‘Reasons for not seeking medical help for severe pelvic floor symptoms: a qualitative study in survivors of gynaecological cancer’
As brain research in pain processing suggests, pain engages overlapping cortical networks responsible for nociception, cognition, emotion, stress and memory, a treatment model targeting nociceptive mechanisms alone can be inadequate to address the complexities of a patient’s pain experience.1 To help physical therapists understand and more effectively address multiple factors influencing a patient’s pain, the APTA, Orthopaedic Section and Pain Management Special Interest Group have brought together 10 physical therapists and a physician from around the country to present an informative and dynamic 2-day pre-conference course, Keep Calm and Treat Pain, Feb 21 and 22 at CSM 2018 in New Orleans. Presentation topics include the Science of Pain, Pain Education, Pain Psychology, Motivational Interviewing and Sleep and Pain. In addition, I will present An Introduction to Mindful Awareness Training and Its Role in Pain Treatment, and my colleague at Herman and Wallace, Megan Pribyl, PT, MSPT, will present Pain and Nutrition: Building Resilience Through Nourishment.
 As we are in the midst of the opioid crisis, this programming could not come at a better time. In this regard, I am especially excited to share information on how mindfulness training has been shown to help patients who are reducing opioid medications to increase positive affect, decrease pain interference and reduce opioid craving.2, 3 I will also describe how mindful awareness training helps address a patient’s fears and fear avoidant behavior and will guide mindfulness exercises.4, 5
As we are in the midst of the opioid crisis, this programming could not come at a better time. In this regard, I am especially excited to share information on how mindfulness training has been shown to help patients who are reducing opioid medications to increase positive affect, decrease pain interference and reduce opioid craving.2, 3 I will also describe how mindful awareness training helps address a patient’s fears and fear avoidant behavior and will guide mindfulness exercises.4, 5
I am honored to be a part of this pioneering program that combines didactic presentations with experiential exercises and lab practice to offer participants the latest science of pain and practical skills to more successfully treat pain. In addition, I am presenting an Educational Session sponsored by the Federal Section on the topic Mindful Awareness Training for Veterans with Comorbid Pain and PTSD based on my research experience at the Puget Sound VA in Seattle. I hope to see you at CSM!
While these presentations offer a taste of mindfulness training to improve patient outcomes, they provide just a glimpse into its potential. My joy and passion is my course, Mindfulness-Based Pain Treatment, where I can offer an in-depth exploration of the role mindful awareness training in pain treatment through a thorough review of mindfulness and pain research, the detailed exploration of the application of mindful awareness training to the biopsychosocial pain model and multiple experiential exercises and lab practices that provide participants with practical strategies to bring into the clinic Monday morning. I hope you can attend a Mindfulness-Based Pain Treatment course offered by Herman and Wallace in 2018 at Samuel Merritt University in Oakland, CA, June 9 and 10, Virginia Hospital Center in Arlington VA Aug 4 and 5, or Pacific Medical Center in Seattle, WA Nov 3 and 4. I look forward to helping you expand your toolbox of treatment techniques for patients with pain conditions.
1. Simons LE, Elman, I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev. 2014;39:61-78.
2. Garland EL, Thomas E, Howard MO. Mindfulness-Oriented Recovery Enhancement ameliorates the impact of pain on self-reported psychological and physical function among opioid-using chronic pain patients. J Pain Symptom Manage. 2014;48(6):1091-9.
3. Garland EL, Froelinger B, Howard MO. Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: exploratory ERP findings from a pilot RTC. J Behav Med. 2015;38(2):327-36.
4. Schutze R, Rees C, Preece M, Schutze M. Low mindfulness predicts pain catastrophizing in fear avoidance model of chronic pain. Pain. 2010; 148(1):120-7.
5. Jay J, Brandt M, Jakobsen MD, et al. Ten weeks of physical-cognitive-mindfulness training reduces fear-avoidance beliefs about work-related activity. Medicine (Baltimore). 2016;95(34):e3945.
Interstitial cystitis is a chronic pain condition characterized by both pelvic pain and urinary symptoms. It’s diagnosed by unexplained pain or pressure that is perceived to be related to the bladder, and affects more than 12 million Americans. It’s often described as the sensation of a urinary tract infection, but without any bacterial infection. Many patients report severe pain, often more intense than that associated with bladder cancer, and up to 85% of patients have accompanying pelvic floor dysfunction.
Pelvic floor physical therapy is the most proven treatment for interstitial cystitis. It’s recommended by the American Urological Association (AUA) as a first-line medical treatment in their IC Guidelines, and is the only treatment given an evidence grade of ‘A’. Furthermore, it’s the sole intervention that provides sustained relief; bladder treatments and oral medications must be continued indefinitely to provide benefit, if they work at all.
Research has demonstrated that at least 85% of patients with interstitial cystitis also have pelvic floor dysfunction. In fact, many of the symptoms of IC can only be explained by the pelvic floor. The majority of patients report painful intercourse, low back pain, hip pain, or constipation accompanying the condition; symptoms that have nothing to do with the bladder.
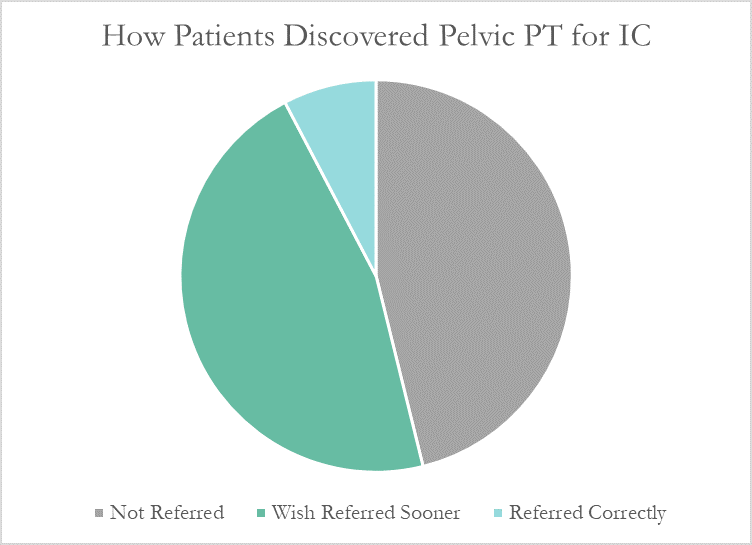
Despite this, many patients don’t learn about pelvic floor physical therapy for years after their diagnosis. Many have to discover pelvic PT for themselves, or their doctor only mentions physical therapy as a last resort. At PelvicSanity, we just published a study of our interstitial cystitis patients in the International Pelvic Pain Society (IPPS) meeting, reporting on both patient outcomes and their experience with the medical system following their IC diagnosis.
In following the results for thirteen consecutive patients with an interstitial cystitis diagnosis, patients reported more than a 60% improvement in pain, symptom bother, and how much symptoms limited their daily activities. On average, their pain level was at a 7.6 out of 10 upon initial evaluation, which fell to 2.6 after treatment.
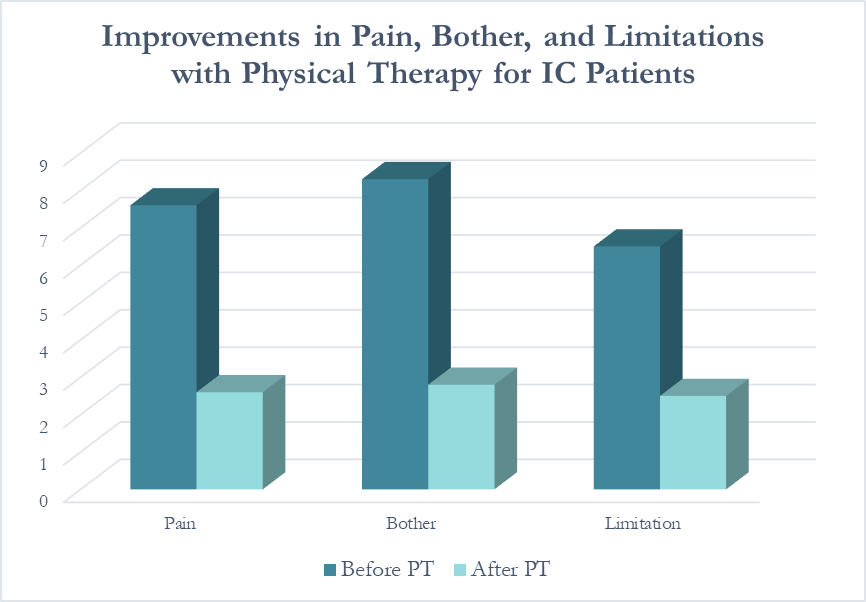 Patients saw a relatively rapid improvement in their symptoms with treatment. Over half (54%) reported an improvement in symptoms within their first three visits; 31% saw their first improvement in visits 4-6 and 15% required ten or more visits for subjective improvement. Importantly, all patients in the study reported a better understanding of their condition and feeling more hopeful for recovery after their initial evaluation.
Patients saw a relatively rapid improvement in their symptoms with treatment. Over half (54%) reported an improvement in symptoms within their first three visits; 31% saw their first improvement in visits 4-6 and 15% required ten or more visits for subjective improvement. Importantly, all patients in the study reported a better understanding of their condition and feeling more hopeful for recovery after their initial evaluation.
More than half of these patients reported seeing five or more medical doctors for their condition prior to beginning pelvic floor physical therapy, and had been prescribed multiple medications and undergone bladder treatments without success. However, only a single respondent (7.7%) believed they had been referred to pelvic PT by their doctor at the appropriate time. Nearly half (46%) had to find out about pelvic floor physical therapy for interstitial cystitis themselves, while the remainder felt they had been referred by their doctor far too late, as a last resort.
With more than 12 million women and men suffering with this condition in the United States alone, increasing education – for both doctors and patients – is vital. In our upcoming course for physical therapists in treating interstitial cystitis (April 28-29, 2018 in San Diego), we’ll focus on the most important physical therapy techniques for IC, home stretching and self-care programs, and information to guide patients in creating a holistic treatment plan