I work at University of Chicago and we are in the throes of preparing for a (big T) Trauma Center. But I am physical therapist who works with (little t) traumatized patients- as I treat only pelvic or oncology patients (and usually both).
From the online dictionary: Trauma is 1. A deeply distressing or disturbing experience (little t trauma) or 2. Physical injury (injury, damage, wound) yes- big T Trauma. In my experience, the Trauma creates the trauma and the body responds in characteristically uncharacteristic ways (more on this later).
 People in distress/trauma-affected do not respond rationally or characteristically, so I have learned to respond to distress/trauma in a rational, ethical, legal and caring manner. Always. Every time. To the best of my ability, and without shame or blame.
People in distress/trauma-affected do not respond rationally or characteristically, so I have learned to respond to distress/trauma in a rational, ethical, legal and caring manner. Always. Every time. To the best of my ability, and without shame or blame.
Let’s talk briefly about Trauma Informed Approach
This is a (person), program, institution or system that:
- Realizes the widespread impact of trauma and understands potential paths for recovery
- Recognizes the signs and symptoms of trauma in clients, families, staff and others affected
- Responds by fully integrating knowledge about trauma into policies, procedures and practices
- Seeks to actively resist retraumatization
The Tenets of Trauma Informed Approach
- Safety
- Trustworthiness and transparency
- Peer support
- Collaboration and mutuality
- Empowerment, voice and choice
- Cultural, historical and gender issues
Trauma Specific Interventions
- Survivors need to be respected, informed, supported, connected, and hopeful- in their recovery
- Interrelation between trauma and symptoms of trauma such as substance abuse, eating disorders, depression, anxiety, musculoskeletal presentation, and acute crisis- including suicidal/homicidal ideations (coordination with other service providers)
- Work in a collaborative way with survivors, families and friends of survivor, and other service providers in a way that will empower survivors
Types of trauma are varied but I usually treat survivors of emotional, verbal, sexual and medical trauma. I have even treated patients who felt traumatized by other pelvic floor physical therapists (again, no judgement). Since most of my clinical experience include sexual and medical trauma survivorship, I try to reframe these experiences as potential Post Traumatic Growth, especially when working with my oncology patients. For my pelvic patients who divulge sexual trauma, I don’t dictate or name anything. I allow the survivor to make the rules and definitions. Survivors of sexual trauma need extra care when treating pelvic floor dysfunction.
First, when treating survivors of sexual trauma: expect ‘characteristically uncharacteristic’ events to occur. These include the psychological/somatic effects of passing out, flashbacks, seizures, tremors, dissociation and other mechanisms of coping with the trauma. Have a plan ready for these patients.
Triaging the survivor to assess their needs, when trauma has been verbalized/disclosed:
- Are you safe right now?
- Do you need medical treatment right now?
- What do you need to feel in control of (PT session/immediately after disclosure of trauma)?
- You have choices in your treatment and in your response to trauma.
- I believe you.
- Lastly, is this a situation for mandated reporting?
After assisting the survivor in their journey towards healing, it is imperative that you take care of yourself. Making healthy boundaries (with patients and others), taking time to decompress, creating healthy ritualistic behaviors, mindfulness/relaxation and somatic release (like yoga, massage or working out) is crucial to successfully treating patients who have experienced trauma and who have shared that trauma experience with you.
Because I use gentle yoga for both my trauma survivors’ treatment and for my own self-care, my new course implements evidenced based trauma sensitive yoga. Additionally, modifications for manual therapy are explored. The class is designed to be informative and experiential while integrating the Trauma Informed Approaches of Safety, Trustworthiness and transparency, Peer support, Collaboration and mutuality, Empowerment, voice and choice and Cultural, historical and gender issues.
Join me in Trauma Awareness for the Pelvic Therapist, next available this March in Albany, NY.
Curing cancer but not addressing life-altering complications can be compared to feeding the homeless on Thanksgiving but turning your back on them the rest of the year. We love hearing positive outcomes of a surgery, but we are not always aware of what happens beyond that. Colorectal cancer is often treated by colectomy, and sometimes the survivor of cancer is left with urological or sexual dysfunction, small bowel obstruction, or pelvic lymphedema.
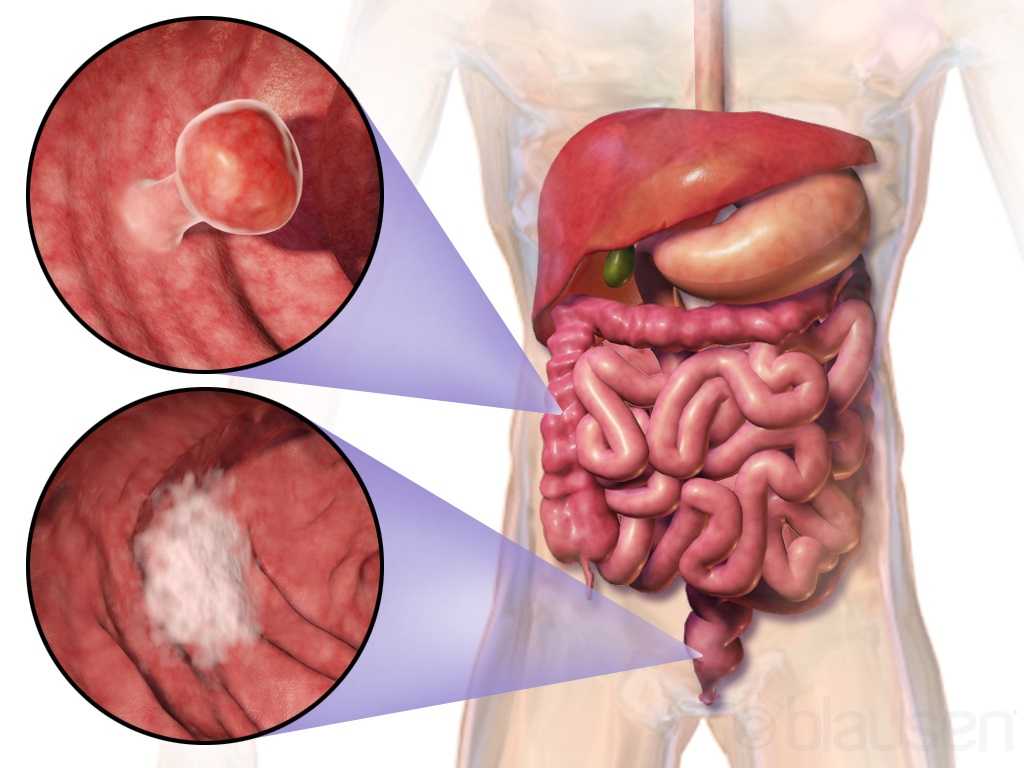 Panteleimonitis et al., (2017) recognized the prevalence of urological and sexual dysfunction after rectal cancer surgery and compared robotic versus laparoscopic approaches to see how each impacted urogenital function. In this study, 49 males and 29 females underwent laparoscopic surgery, and 35 males and 13 females underwent robotic surgery. Prior to surgery, 36 men and 9 women were sexually active in the first group and 13 men and 4 women were sexually active in the latter group. Focusing on the male results, male urological function (MUF) scores were worse pre-operatively in the robotic group for frequency, nocturia, and urgency compared to the laparoscopic group. Post-operatively, urological function scores improved in all areas except initiation/straining for the robotic group; however, the MUF median scores declined in the laparoscopic group. Regarding male sexual function (MSF) scores for libido, erection, stiffness for penetration and orgasm/ ejaculation, the mean scores worsened in all areas for the laparoscopic group but showed positive outcomes for the robotic group. In spite of limitations of the study, the authors concluded robotic rectal cancer surgery may afford males and females more promising urological and sexual outcomes as robotic.
Panteleimonitis et al., (2017) recognized the prevalence of urological and sexual dysfunction after rectal cancer surgery and compared robotic versus laparoscopic approaches to see how each impacted urogenital function. In this study, 49 males and 29 females underwent laparoscopic surgery, and 35 males and 13 females underwent robotic surgery. Prior to surgery, 36 men and 9 women were sexually active in the first group and 13 men and 4 women were sexually active in the latter group. Focusing on the male results, male urological function (MUF) scores were worse pre-operatively in the robotic group for frequency, nocturia, and urgency compared to the laparoscopic group. Post-operatively, urological function scores improved in all areas except initiation/straining for the robotic group; however, the MUF median scores declined in the laparoscopic group. Regarding male sexual function (MSF) scores for libido, erection, stiffness for penetration and orgasm/ ejaculation, the mean scores worsened in all areas for the laparoscopic group but showed positive outcomes for the robotic group. In spite of limitations of the study, the authors concluded robotic rectal cancer surgery may afford males and females more promising urological and sexual outcomes as robotic.
Husarić et al., (2016) considered the risk factors for adhesive small bowel obstruction (SBO) after colorectal cancer colectomy, as SBO is a common morbidity that causes a decrease in quality of life. They performed a retrospective study of 248 patients who underwent colon cancer surgery, and 13.7% of all the patients had SBO. Thirty (14%) of the 213 males and 9 (12.7%) of the 71 females had SBO; consequently, they found patients being >60 years old was a more significant risk factor than sex regarding occurrence of SBO. The authors concluded a Tumor-Node Metastasis stage of >3 and immediate postoperative complications were found to be the greatest risk factors for SBO.
Vannelli et al., (2013) explored the prevalence of pelvic lymphedema after lymphadenectomy in patients treated surgically for rectal cancer. Five males and 8 females were examined one week before and 12 months after being discharged from the hospital. All 9 of the patients (4 males, 5 females) with extra-peritoneal cancer exhibited lymphedema via MRI, but the 4 (1 male, 3 females) patients with intra-peritoneal cancer had none. The authors concluded pelvic lymphedema can be elusive after rectal surgery, but pelvic disorders persist and patients should be routinely examined for it.
Obviously saving a life is the primary goal when it comes to cancer. But just like caring for the destitute for one day doesn’t cure a lifetime of hunger, ignoring the negative post-surgical sequelae of a colectomy prevents a cancer survivor from living a healthy life. Herman & Wallace offers two pelvic floor oncology courses, “Oncology and the Male Pelvic Floor” and "Oncology and the Female Pelvic Floor" , which address how pelvic cancers affect the quality of life of our patients and how practitioners can make a positive impact.
Panteleimonitis, S., Ahmed, J., Ramachandra, M., Farooq, M., Harper, M., & Parvaiz, A. (2017). Urogenital function in robotic vs laparoscopic rectal cancer surgery: a comparative study. International Journal of Colorectal Disease, 32(2), 241–248. http://doi.org/10.1007/s00384-016-2682-7
Husarić E., Hasukić Š, Hotić N, Halilbašić A, Husarić S, Hasukić I. (2016). Risk factors for post-colectomy adhesive small bowel obstruction. Acta
“Keep Calm and Treat Pain” is perhaps an affirmation for therapists when encountering patients suffering from pain, whether acute or chronic. The reality is this: treating pain is complicated. Treating pain has brought many a health care provider to his or her proverbial knees. It has also led us as a nation into the depths of the opioid epidemic which claimed over 165,000 lives between the years of 1999 and 2014 (Dowell & Haegerich, 2016). That number has swollen to over 200,000 in up-to-date calculations and according to the CDC, 42,000 human beings, not statistics, were killed by opioids in 2016 - a record.
 So why has treating pain eluded us as a nation? The answers are as complicated as treating pain itself. Which is why we as health care providers must seek out not simply alternatives, but the truth in the matter. Why are so many suffering? Why has chronic pain become the enormous beast that it has become? What might we do differently, collectively, and how might we examine this issue through a holistic mindset?
So why has treating pain eluded us as a nation? The answers are as complicated as treating pain itself. Which is why we as health care providers must seek out not simply alternatives, but the truth in the matter. Why are so many suffering? Why has chronic pain become the enormous beast that it has become? What might we do differently, collectively, and how might we examine this issue through a holistic mindset?
In just a few weeks, I have the privilege of teaching amongst 10 physical therapy professionals and one physician from around the nation who with coordinated efforts created a landmark pre-conference course at CSM in New Orleans through the Orthopaedic Section of the APTA. Included in the 11 are myself and another Herman & Wallace instructor Carolyn McManus, PT, MS, MA who teaches “Mindfulness Based Pain Treatment” through the Institute.
The CSM pre-conference course title is “Keep Calm and Treat Pain” representing a necessary effort to provide the clinician with ideas and inspiration for helping the profession as a whole treat pain with an integrative approach.
“Pain and Nutrition: Building Resilience Through Nourishment” is the section I look forward to sharing. It will introduce concepts we can leverage to allow us confidence in seeking alternate ways of taming this beast which is chronic pain - ways which can enhance health and well-being of our clients in pelvic rehabilitation. We must not be passive observers of the opioid epidemic. We must come to terms with the fact that our nations go-to tool for treating pain unfortunately causes side-effects which can and does include loss of life. We can do better. And we will.
While the CSM pre-conference course will give you a taste of the nutrition concepts available to you, it is a mere tip of the nourishment iceberg. I continue my passion and mission with the two-day course titled “Nutrition Perspectives for the Pelvic Rehab Therapist”, an experience that can elevate your conversations with clients. It will pave a path of understanding for the provider, allowing us to share options, understanding, and hope. “Nutrition Perspectives for the Pelvic Rehab Therapist is coming next to Maywood, IL March 3 & 4, 2018. I welcome you to join me.
APTA CSM: https://apta.expoplanner.com/index.cfm?do=expomap.sess&event_id=27&session_id=13763. Accessed January 8, 2018.
CDC: https://www.cdc.gov/drugoverdose/index.html. Accessed January 8, 2018.
Dowell, D., & Haegerich, T. M. (2016). Using the CDC Guideline and Tools for Opioid Prescribing in Patients with Chronic Pain. Am Fam Physician, 93(12), 970-972.
Lerner, A., Neidhofer, S., & Matthias, T. (2017). The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists. Microorganisms, 5(4). doi:10.3390/microorganisms5040066
Murthy, V. H. (2016). Ending the Opioid Epidemic - A Call to Action. N Engl J Med, 375(25), 2413-2415. doi:10.1056/NEJMp1612578
While my dad was visiting Michigan, we had the day to ourselves as my kids were in school. I was so excited to have quality time with my dad. Unfortunately it was pouring down rain. We decided on a leisurely brunch and then a movie. Dad chose the movie, “Wind River.” While not a movie I would normally pick, I was happy to go along. A little more than half way through…there was a horribly violent scene against a young women. I panicked, plugged my ears and closed my eyes. Unfortunately some images were burned into the back of my mind. When the movie was over, I remained seated and tears just came. My dad held me while I cried. I was able to calm down and leave the theater, but the images continued to bother me. During the next few days, I made it a priority to care for myself and allow my nervous system to process and heal.
 What happened to me? I have never had any traumatic personal experience. Why did I react so strongly? I talked with my therapist about it and she suggested I might have experienced secondary traumatic stress. We know, as pelvic health therapists, we need extra time to hear the “stories” of new patients. We do our best to create a safe space for them so they can trust us and we can help them discover pathways to healing. Yet no one has taught us what we are supposed to do with the traumatic stories our patients share. How are we to cope with holding space for their pain? How do we put on a happy face as we exit the room to get the next patient?
What happened to me? I have never had any traumatic personal experience. Why did I react so strongly? I talked with my therapist about it and she suggested I might have experienced secondary traumatic stress. We know, as pelvic health therapists, we need extra time to hear the “stories” of new patients. We do our best to create a safe space for them so they can trust us and we can help them discover pathways to healing. Yet no one has taught us what we are supposed to do with the traumatic stories our patients share. How are we to cope with holding space for their pain? How do we put on a happy face as we exit the room to get the next patient?
Teaching Capstone over the last few years, Nari Clemons and I have talked with many of you who were feeling emotionally overloaded especially when treating chronic pelvic pain and trauma survivors. Some of you were experiencing job burnout, others were deciding maybe it was time for a career shift, away from the pelvis. We realized something needed to be done as our field was losing talented pelvic health therapists. We have also struggled ourselves with various aspects of our profession.
There are no studies that directly look at job burn out, secondary traumatic stress, and compassion fatigue among pelvic health physical therapists. Yet these problems are common among social workers, physicians and other people groups in health care. There are individual as well as institutional risk factors that lead to the development of each. The solution, as one self-help module puts it, is developing resilience. A large part of this skill is making self-care a priority. The basics such as adequate sleep, nutrition, and exercise are foundational. Meditation, mindfulness, therapy, and spiritual practices, as well as supportive friends/groups are also imperative.
Nari and I realized that training to develop resilience in therapists was missing. Initially we equipped ourselves to have better boundaries, ground ourselves with meditation, mindfulness and exercise, which enhanced our skills in dealing with complex, chronic patients. We compiled what we have learned and want to share it with you. We would like to invite you to attend Holistic Interventions and Meditation: Boundaries, Self-Care, and Dialogue. We have designed this 3 day course to be partially educational and absolutely experiential. We are going to dig deeper into ways to calm our patient’s and our own nervous systems, explore and practice the latest recommendations on treatment of persistent pain, we will mediate and learn about mediation, play with essential oils, learn some new hands on techniques, and support and encourage one another as we build communication skills. We want you to leave feeling refreshed and equipped to continue to treat patients without losing yourself in the process. We want to invest in you so you can continue the investment you have made in your career and avoid job burnout, compassion fatigue and secondary trauma. We invite you to develop the resilience you need for a rewarding career in pelvic health physical therapy by joining us in Tampa this January.
Cieslak, R., Shoji, K., Douglas, A., Melville, E., Luszczynska, A., & Benight, C. C. (2014). A meta-analysis of the relationship between job burnout and secondary traumatic stress among workers with indirect exposure to trauma. Psychological services, 11(1), 75.
Meadors, P., Lamson, A., Swanson, M., White, M., & Sira, N. (2010). Secondary traumatization in pediatric healthcare providers: Compassion fatigue, burnout, and secondary traumatic stress. OMEGA-Journal of Death and Dying, 60(2), 103-128.
Sodeke-Gregson, E. A., Holttum, S., & Billings, J. (2013). Compassion satisfaction, burnout, and secondary traumatic stress in UK therapists who work with adult trauma clients. European journal of psychotraumatology, 4(1), 21869.
Stearns, S., & Benight, C. C. (2016). Organizational Factors in Burnout and Secondary Traumatic Stress. In Secondary Trauma and Burnout in Military Behavioral Health Providers (pp. 85-113). Palgrave Macmillan US.
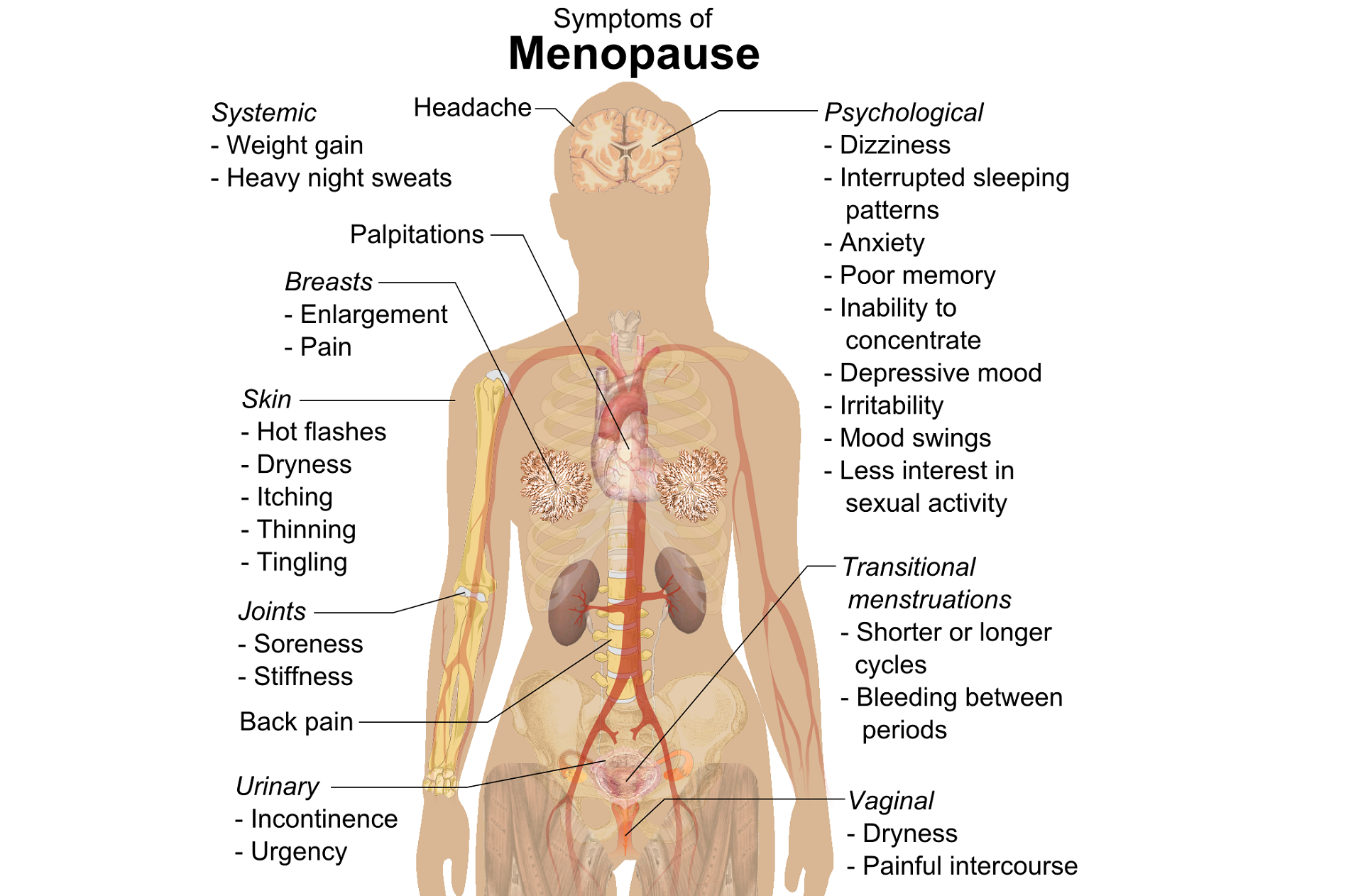
The new year is here and with it, lots of motivational posting about exercise and weight loss…but how is this desire for ‘new year, new you’ affecting peri-menopausal women with urinary dysfunction? It has been established that the lower urinary tract is sensitive to the effects of estrogen, sharing a common embryological origin with the female genital tract, the urogenital sinus. Urge urinary incontinence is more prevalent after the menopause, and the peak prevalence of stress incontinence occurs around the time of the menopause (Quinn et al 2009). Zhu et al looked at the risk factors for urinary incontinence in women and found that some of the main contributors include peri/post-menopausal status, constipation and central obesity (women's waist circumference, >/=80 cm) along with vaginal delivery/multiparity.
 Could weight loss directly impact urinary incontinence in menopausal women? In a word – yes. ‘Weight reduction is an effective treatment for overweight and obese women with UI. Weight loss of 5% to 10% has an efficacy similar to that of other nonsurgical treatments and should be considered a first line therapy for incontinence’ (Subak et al 2005) But do these benefits last? Again – yes! ‘Weight loss intervention reduced the frequency of stress incontinence episodes through 12 months and improved patient satisfaction with changes in incontinence through 18 months. Improving weight loss maintenance may provide longer term benefits for urinary incontinence.’ (Wing et al 2010)
Could weight loss directly impact urinary incontinence in menopausal women? In a word – yes. ‘Weight reduction is an effective treatment for overweight and obese women with UI. Weight loss of 5% to 10% has an efficacy similar to that of other nonsurgical treatments and should be considered a first line therapy for incontinence’ (Subak et al 2005) But do these benefits last? Again – yes! ‘Weight loss intervention reduced the frequency of stress incontinence episodes through 12 months and improved patient satisfaction with changes in incontinence through 18 months. Improving weight loss maintenance may provide longer term benefits for urinary incontinence.’ (Wing et al 2010)
The other major health issues facing women at midlife include an increased risk for cardiovascular disease, Type 2 Diabetes and Bone Health problems – all of which are responsive to lifestyle interventions, particularly exercise and stress management. In their paper looking at lifestyle weight loss interventions, Franz et al found that ‘…a weight loss of >5% appears necessary for beneficial effects on HbA1c, lipids, and blood pressure. Achieving this level of weight loss requires intense interventions, including energy restriction, regular physical activity, and frequent contact with health professionals’. 5% weight loss is the same amount of weight loss necessary to provide significant benefits for urinary incontinence at midlife.
Successful weight management depends on nutritional intake, exercise and psychosocial considerations such as stress management, but for the menopausal woman, hormonal balance can also have an effect on not only bladder and bowel dysfunction but changing metabolic rates, thyroid issues and altered weight distribution patterns. As pelvic rehab therapists, we are all aware that pelvic health issues can be a barrier to exercise participation but sensitive awareness of the other particular challenges facing midlife women can make the difference in developing a beneficial therapeutic alliance and a journey back to optimal health. If you would like to explore the topics surrounding optimal health at menopause, why not join me in California in February?
Climacteric. 2009 Apr;12(2):106-13. ‘The effects of hormones on urinary incontinence in postmenopausal women.’ Quinn SD, Domoney C. Menopause. 2009 Jul-Aug;16(4):831-6. The epidemiological study of women with urinary incontinence and risk factors for stress urinary incontinence in China’ Zhu L, Lang J, Liu C, Han S, Huang J, Li X. J Urol. 2005 Jul;174(1):190-5. Weight loss: a novel and effective treatment for urinary incontinence’ Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. J Urol. 2010 Sep;184(3):1005-10. Effect of weight loss on urinary incontinence in overweight and obese women: results at 12 and 18 months Wing RR, West DS, Grady D, Creasman JM, Richter HE, Myers D, Burgio KL, Franklin F, Gorin AA, Vittinghoff E, Macer J, Kusek JW, Subak LL; Program to Reduce Incontinence by Diet and Exercise Group. J Acad Nutr Diet. 2015 Sep;115(9):1447-63. doi: 10.1016/j.jand.2015.02.031. Epub 2015 Apr 29. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lean, M, & Lara, J & O Hill, J (2007) Strategies for preventing obesity. In: Sattar, N & Lean, M (eds.) ABC of Obesity. Oxford, Blackwell Publishing.
When I work prn in inpatient rehabilitation, I have access to each patient’s chart and can really focus on the systems review and past medical history, which often gives me ample reasons to ask about pelvic floor dysfunction. So, of course, I do. I have yet to find a gynecological cancer survivor who does not report an ongoing struggle with urinary incontinence. And sadly, they all report that they just deal with it.
 Bretschneider et al.2016 researched the presence of pelvic floor disorders in females with presumed gynecological malignancy prior to surgical intervention. Baseline assessments were completed by 152 of the 186 women scheduled for surgery. The rate of urinary incontinence (UI) at baseline was 40.9% for the subjects, all of whom had uterine, ovarian, or cervical cancer. Stress urinary incontinence (SUI) was reported by 33.3% of the women, urge incontinence (UI) by 25%, fecal incontinence (FI) by 3.9%, abdominal pain by 47.4%, constipation by 37.7%, and diarrhea by 20.1%. The authors concluded pelvic floor disorders are prevalent among women with suspected gynecologic cancer and should be noted prior to surgery in order to provide more thorough rehabilitation for these women post-operatively.
Bretschneider et al.2016 researched the presence of pelvic floor disorders in females with presumed gynecological malignancy prior to surgical intervention. Baseline assessments were completed by 152 of the 186 women scheduled for surgery. The rate of urinary incontinence (UI) at baseline was 40.9% for the subjects, all of whom had uterine, ovarian, or cervical cancer. Stress urinary incontinence (SUI) was reported by 33.3% of the women, urge incontinence (UI) by 25%, fecal incontinence (FI) by 3.9%, abdominal pain by 47.4%, constipation by 37.7%, and diarrhea by 20.1%. The authors concluded pelvic floor disorders are prevalent among women with suspected gynecologic cancer and should be noted prior to surgery in order to provide more thorough rehabilitation for these women post-operatively.
Ramaseshan et al.2017 performed a systematic review of 31 articles to study pelvic floor disorder prevalence among women with gynecologic malignant cancers. Before treatment of cervical cancer, the prevalence of SUI was 24-29% (4-76% post-treatment), UI was 8-18% (4-59% post-treatment), and FI was 6% (2-34% post- treatment). Cervical cancer treatment also caused urinary retention (0.4-39%), fecal urge (3-49%), dyspareunia (12-58%), and vaginal dryness (15-47%). Uterine cancer showed a pre-treatment prevalence of SUI (29-36%), UUI (15-25%), and FI (3%) and post-treatment prevalence of UI (2-44%) and dyspareunia (7-39%). Vulvar cancer survivors had post-treatment prevalence of UI (4-32%), SUI (6-20%), and FI (1-20%). Ovarian cancer survivors had prevalence of SUI (32-42%), UUI (15-39%), prolapse (17%) and sexual dysfunction (62-75%). The authors concluded pelvic floor dysfunction is prevalent among gynecologic cancer survivors and needs to be addressed.
Lindgren, Dunberger, & Enblom2017 explored how gynecological cancer survivors (GCS) relate their incontinence to quality of life, view their physical activity/exercise ability, and perceive pelvic floor muscle training. The authors used a qualitative interview content analysis study with 13 women, age 48–82. Ten women had UI and 3 had FI after treatment (2 had radiation therapy, 5 had surgery, and 6 had surgery as well as radiation therapy). The results showed a reduction in physical and psychological quality of life and sexual activity because of incontinence. Having minimal to no experience or even awareness of pelvic floor training, 9 out of the 10 women were willing to spend 7 hours a week to improve their incontinence. Practical and emotional coping strategies also helped these women, and they all declared they had the cancer treatments without being informed of the risk of incontinence, which impacted their attitude and means of handling the situation.
Research shows incontinence is a common occurrence after gynecological cancer treatment. It impacts quality of life after surviving a serious illness, and many women do not know pelvic floor therapy can improve their situation. Oncology and the Female Pelvic Floor is an ideal course for practitioners to take to help increase their knowledge on how to educate and treat this population.
Bretschneider, C. E., Doll, K. M., Bensen, J. T., Gehrig, P. A., Wu, J. M., & Geller, E. J. (2016). Prevalence of pelvic floor disorders in women with suspected gynecological malignancy: a survey-based study. International Urogynecology Journal, 27(9), 1409–1414. http://doi.org/10.1007/s00192-016-2962-3
Ramaseshan, A.S., Felton, J., Roque, D., Rao, G., Shipper, A.G., Sanses, T.V.D. (2017). Pelvic floor disorders in women with gynecologic malignancies: a systematic review. International Urogynecology Journal. http://doi.org/10.1007/s00192-017-3467-4
Lindgren, A., Dunberger, G., & Enblom, A. (2017). Experiences of incontinence and pelvic floor muscle training after gynaecologic cancer treatment. Supportive Care in Cancer, 25(1), 157–166. http://doi.org/10.1007/s00520-016-3394-9
Perimenopausal pelvic health issues are, for many of us, some of the most common issues that we see in the women that we work with. Urinary incontinence is one of the most important issues for peri- and postmenopausal women. In Melville’s study1 of U.S. women, half of the participants between the ages of 50 and 90 experienced urine leakage every month. Zhu’s 2008 study2 looked at the risk factors for SUI - Multiple vaginal deliveries, Age/postmenopausal status, Chronic pelvic pain, Obesity, lack of exercise, constipation, and hypertension. But what is not often (enough) looked at in the research, is the link between urinary dysfunction and sexual dysfunction – usually because questions aren’t asked or assumptions are made. In Mestre et al’s 2015 paper3, they write ‘…Integrating sexual health in clinical practice is important. In women with pelvic floor disorders, the evaluation of the anatomical defects, lower urinary tract function and the anorectal function often receives more attention than sexual function.’
 But are they linked?
But are they linked?
In Moller’s exploration of this topic, they report that lower urinary tract symptoms (LUTS) have a profound impact on women’s physical, social, and sexual wellbeing. Unsurprisingly (to pelvic rehab specialists at least!), they found that the LUTS are likely to affect sexual activity. Conversely, sexual activity may affect the occurrence of LUTS. The aims of the Moller study were to elucidate to which extent LUTS affect sexual function and to which extent sexual function affect LUTS in an unselected population of middle-aged women in 1 year. A questionnaire was sent to 4,000 unselected women aged 40–60 years. Compared to women having sexual relationship, a statistically significant 3 to 6 fold higher prevalence of LUTS was observed in women with no sexual relationship. In women who ceased sexual relationship an increase in the de novo occurrence of most LUTS was observed. In women who resumed sexual relationship a decrease in LUTS was observed. In women whose sexual activity was unchanged no change in the occurrence of LUTS. So they rightfully concluded ‘…sexual inactivity may lead to LUTS and vice versa.’
In my Menopause course, we will explore the range of perimenopausal pelvic health issues that many women face and their inter-related nature – not just with each other but also how orthopaedic, endocrine and gastro-intestinal health issues influence pelvic health and wellness. Interested in learning more? Come and join the conversation in California in February 2018!
1. Melville JL, et al. Urinary incontinence in US women: a population-based study. Arch Intern Med 2005;165(5):537-42 - See more at: http://www.nursingcenter.com/lnc/JournalArticle?Article_ID=698029#sthash.cm8A90tS.dpuf
2. Zhu L1, Lang J, Wang H, Han S, Huang J. Menopause. 2008 May-Jun;15(3):566-9. The prevalence of and potential risk factors for female urinary incontinence in Beijing, China
3. Mestre M, Lleberia J, Pubill J, Espuña-Pons M Actas Urol Esp. 2015 Apr;39(3):175-82. Epub 2014 Aug 28. Questionnaires in the assessment of sexual function in women with urinary incontinence and pelvic organ prolapse.
Recently my coworkers and I celebrated a male patient’s recovery from a long and difficult journey with persistent pelvic pain. “Ben’s” case had many elements of what we normally see in our patients: chronic muscle holding, restricted fascia, allodynia, hyperalgesia, castrophizing and kenisiophobia. Ben was also very upfront about how his pain impacted his emotional well-being and vice versa. His healing process taught us a lot about the biopsychosocial aspects of treating persistent pain. Along his journey we dreamed of the day we could write a blog together and help other people learn from the experience. Ben also decided to make a career change entering school to become a PTA so that he could help others in pain. Here is my interview with this brave patient.
 1. Tell us about how your pain started
1. Tell us about how your pain started
My pain started with urethral burning. Tests showed there was no infection. In retrospect, the cause of pain could have been the beginning of tension on pudendal nerve branches from extreme stress and a series of traumatic incidents that happened within weeks of each other. They included a very embarrassing and stressful summer of unemployment, a father who had heart failure and triple bypass in the fall, and a girlfriend who gave me an ultimatum when I was too stressed to get an erection.
2. What medical tests or treatments were done?
When the pain started, I first thought it was a basic urinary tract infection. I went to the med center and was prescribed an antibiotic. After 3 days without change, I went back in and although they still found no sign of infection, they prescribed an additional antibiotic. The urethral pain never stopped and seemed to get worse. Following a series of visits to numerous doctors and urologists, I repeated tests on the prostate fluid, blood tests, and more bacterial tests. No infection. My PCP also made a fairly large overture of testing me repeatedly for HIV. For five months I had a blood test every month, all came back negative. This was damaging to my psyche. For those months I was terrified my life was over. In retrospect, that doctor was out of line, I changed doctors.
3. What were your thoughts when your doctor suggested physical therapy?
When the doctor suggested pelvic floor physical therapy, I was a little skeptical because I was still convinced that something was wrong in a chemical or infectious way (as is typical for most men with pelvic floor dysfunction). However, desperate to take away the constant pain, I followed the advice.
Stay tuned for part two of our conversation with Ben, coming up in our next post on the Pelvic Rehab Report!
Today we pick up on Jennafer Vande Vegte's interview with her patient, "Ben", about his experience overcoming chronic pelvic pain syndrome. Ben's quality of life improved so much that he has returned to school in order to become a PTA, with a focus on pelvic rehabilitation!
Describe your physical therapy experience. Talk about your recovery process. Include the physical, mental and emotional components.
 For my initial visit, my therapist questioned and assessed my pain, then explained pelvic floor dysfunction. She made sure I understood that the evaluation and treatment process involved internal rectal work. After developing the condition and months of seeing doctors who didn’t listen, finally I found a physical therapist who was actually listening to me and determined to get to the bottom of what was going on. I could tell she already knew much about the mechanics (if not the exact cause) because she had treated other patients with the same issues. I immediately sensed a difference from any other health care professional in attitude, compassion, and knowledge. Of course, how do you know for sure? Well, you don’t. But after repeated visits and excellent results, you experience the difference. An important realization while going to Physical Therapy is learning to see the mind-body connection. In the back of my mind I sensed that my pain was being perpetuated by emotional trauma. This is not an intuitive way of thinking when you are in constant high-level, 5-alarm pain. I was obsessed with finding the cause of my pain, but chronic pain is extremely elusive and complicated.
For my initial visit, my therapist questioned and assessed my pain, then explained pelvic floor dysfunction. She made sure I understood that the evaluation and treatment process involved internal rectal work. After developing the condition and months of seeing doctors who didn’t listen, finally I found a physical therapist who was actually listening to me and determined to get to the bottom of what was going on. I could tell she already knew much about the mechanics (if not the exact cause) because she had treated other patients with the same issues. I immediately sensed a difference from any other health care professional in attitude, compassion, and knowledge. Of course, how do you know for sure? Well, you don’t. But after repeated visits and excellent results, you experience the difference. An important realization while going to Physical Therapy is learning to see the mind-body connection. In the back of my mind I sensed that my pain was being perpetuated by emotional trauma. This is not an intuitive way of thinking when you are in constant high-level, 5-alarm pain. I was obsessed with finding the cause of my pain, but chronic pain is extremely elusive and complicated.
Over the course of many months of PT though we couldn’t pinpoint what started the pain, we knew my nervous system was keeping it going. Sensory signals had somehow been rerouted through pain centers in the delicate and complicated highway interstate of the nervous system. It was as if the Fed Ex truck that was supposed to carry a package from Miami to Denver got rerouted to New York, stuck in traffic in Manhattan, flipped off by cab drivers, beaten up by gang members, contents of the truck shaken up by the driver trying to flee the city, and then finally finding the way out of New York to the true destination of Denver – with damaged goods, and shaking with anxiety. As to who the idiot dispatcher was who re-routed the truck to New York, well, he’s really good at keeping himself secret and innocent-looking. Jerk!
Physical therapy, over time, began to work for me. It released trigger points which are the first step in the long process of recovery. As we know, chronic tension must be addressed in tissues and nerves, and the mind must relearn how to remain in neutral. I found that as I gained periods of relief I could see that there truly was a mind-body connection beyond what I could imagine. My physical therapist and I both knew that nerves are the slowest recovering tissue in the body, and when you combine that with an anxious mind, you have a complicated puzzle to solve. There is definitely a closed circuit that develops with chronic pelvic pain. Pain causes anxiety, anxiety causes pain and circularly they feed one another.
During my physical therapy I joined a male pelvic pain message board online. I began understanding that most men who develop pelvic pain also have experienced traumatic emotional stress. And a large part of chronic pelvic pain is rooted in a mind-body dysfunction. I had to learn how to stop thinking catastrophically, especially during flare ups. I had to trust that my body would heal and think positively. I had to learn how to relax, take care of myself, eat well, stretch and exercise daily.
When I started physical therapy, I hoped to escape the pain. My first 5 month phase of physical therapy helped to loosen the chronically tightened pelvic sphincter muscles. However, I still had allodynia. In my second phase of physical therapy I began experiencing reduction of pain for a longer duration of time. After about a year of therapy, I finally got to a point where I could see there was significant improvement, even though some intermittent pain and anxious symptoms stubbornly persisted. In late spring of 2017, I finally felt like I had conquered the pain by 98%. Occasionally flares would still come, but they were brief and nothing like before physical therapy.
How has your experience with chronic pelvic pain changed you?
CPPS has profoundly changed me. I don’t take the little things for granted or sweat them anymore. I am grateful for not feeling that horrible, hellish sensation any longer. I appreciate having my mind pain and panic free. I speak my mind while respecting my own desires instead of belittling them. I am currently in school to become a Physical Therapy Assistant as through this process I learned that I’m actually much smarter than my middle school guidance counselor thought. I understand the mind is incredibly powerful, and fear rarely has the same power over me.
How do you handle flare ups?
I now handle brief flare ups with deep breaths, meditation, and/or just taking a step back and trying to zero in on what is really bothering me. At least now I can clearly think without debilitating pain and am able to function.
What would you like to say to other people who are struggling with chronic pelvic pain?
Oh, man. For the initial duration, I would say find a safe place where you can feel as comfortable as possible until the pain lessens. When it is bad, you sort of have to give in to it. However, part of this recovery is the physical mechanics of muscle and fascia. Physical Therapy is essential in the process of recovery to release this tension. I would tell them not to give up hope. You will not find many health professionals or websites that will tell you that you can beat this and recover 100%. But I will tell you, you can recover, 100%. You can. But for now, your full-time job is to work on recovery, and that includes lots of self-care, facing possible emotional pain, and physical therapy.
If you would like to learn more about addressing the mind body connection with patients please join us for Holistic Intervention and Meditation: Boundaries, Self-Care and Dialog in January. We will be exploring ways to help our patients heal to their fullest potential as well as keeping ourselves emotionally healthy in the process. Treating patients with persistent pain can be challenging for the best of us. Please come for this three-day course where you will leave feeling refreshed, renewed and reinvigorated to treat even your most complex patient.
Additional resources:
https://www.tamethebeast.org/#home
https://www.youtube.com/watch?v=jIsF8CXouk8
http://www.sciencedirect.com/science/article/pii/S1356689X11000737
http://www.noigroup.com/en/Home
https://bodyinmind.org/who-are-we/
Mindful eating requires slowing down and paying attention to the present moment experience of eating. Rather than mindlessly put food into your mouth and not really taste what you’re eating, you deliberately notice the appearance, smell, texture and taste of the food and pay attention to your thoughts, feelings, and physical sensations. Eating mindfully can interrupt habitual eating behaviors and promote greater self-regulation of food choices.1 Warren and colleagues conclude mindful eating has the potential to help address maladaptive eating behaviors and the difficulties many face with controlling food intake.2
 Although mindful breathing, body scan and movement are the core skills I teach patients with persistent pain, I introduce mindful eating as another strategy to cultivate present moment awareness. Patients can have surprising shifts in their relationship to food and frequently comment, “If I ate more mindfully, I would enjoy my food more and eat less!”
Although mindful breathing, body scan and movement are the core skills I teach patients with persistent pain, I introduce mindful eating as another strategy to cultivate present moment awareness. Patients can have surprising shifts in their relationship to food and frequently comment, “If I ate more mindfully, I would enjoy my food more and eat less!”
Lucie Khadduri, PT, DPT, PRPC clinician and Adjunct Professor at the University of Puget Sound School of Physical Therapy, took my course last spring and describes her patient’s experience with mindful eating:
I have been meaning to email for some time to thank you for the April 2017 course on Mindfulness for Rehab Professionals. Your class really impacted my daily PT practice in a positive way. I wanted to share with you one story in particular to illustrate the power that these new tools you have given me have helped others.
I have this male patient who is about 35 years old who struggled with chronic constipation, bloating and anxiety related to his intense fecal urges that were then followed by an inability to defecate. When he started PT, he had just left his job and took a job working from home just so that he could have consistent, stress free bathroom access.
I spoke to him about diaphragmatic breathing and mindfulness and its impact on the autonomic nervous system. What helped him the most, though, was the mindful eating exercise. He has since started applying these concepts to when he eats. He told me on his discharge visit that in the past, he would eat 4 slices of pizza very quickly, without thinking about it and then have horrible pain afterward. Now, he says it is easy to eat 1 slice and have a salad not because he knows salad is better for him, but because his mouth and mind crave different textures and colors in his food. Mindful eating gave him the ability to slow down, focus on the physical sensations of eating and he found that this has changed his relationship with food. As a result, his constipation is much better managed and his anxiety and stress are much better.
Thanks again for an excellent class. I often encourage patients to go to your website for your free 10 minute meditations.
Thank you, Lucie, for sharing this story. It reflects one of the many ways patients benefit from training in mindful awareness. I look forward to introducing colleagues to mindful eating and additional experiential mindful exercises and current research in my upcoming class, Mindfulness-Based Pain Treatment, at Loyola University Stritch School of Medicine, Maywood, Il, September 30 and October 1.
1. Miller CK. Mindful eating with diabetes. Diabetes Spectr. 2017 May;30(2):89-94.
2. Warren JM, Smith N, Ashwell M. A structured literature review on the role of mindfulness, mindful eating and intuitive eating in changing eating behaviors: effectiveness and associated potential mechanisms. Nutr Res Rev. 2017 Jul 18:1 – 12.
Image courtesy of California Institute of Technology