Leg length discrepancy (LLD) is when there is a noticeable difference in length of one leg to the other. LLD is common and can be found in 70% of the population (Gurney, 2002). LLD can be structural or functional. Structural LLD is when a long bone in the leg is longer or shorter than the other. Structural LLD is often the result of congenital or boney damage of epiphyseal plate. Functional is when there is an apparent LLD from higher in the chain such as scoliosis. Generally as pelvic floor therapists we are orthopedic based therapists. In physical therapy school we learned that a leg length discrepancy had to be >1 cm to be considered significant, and based off of recent research that is still the case. Research in the last few years has focused on whether LLD has an effect on age related changes with osteoarthritis, posture & gait, and pain. Physiopedia suggests differential diagnosis of sacroiliac dysfunction, scoliosis, low back pain, iliotibial band (ITB) syndrome, stress fractures, and pronation. It can often feel like a chicken or egg question.
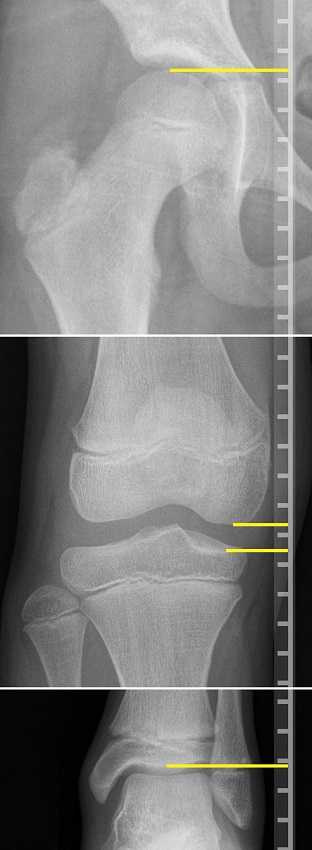 In the clinic I typically screen for a leg length discrepancy during my initial evaluation. A LLD may be noticed upon observation of gait assessment, standing posture, or part of the pelvic obliquity screen in standing and then in supine.
In the clinic I typically screen for a leg length discrepancy during my initial evaluation. A LLD may be noticed upon observation of gait assessment, standing posture, or part of the pelvic obliquity screen in standing and then in supine.
During gait, a LLD will create bilaterteral gait impairments. Khamis et al did a systematic review of LLD and gait deviations in 2017. They narrowed the search down to 12 articles and found that LLD >1cm was significantly related to gait deviations. These deviations occurred bilaterally, and while initially compensations occurred in the sagittal plane, as the LLD increased so did the gait deviations, and then affected frontal planes of motion as well. Resende et al (2016) agrees that even mild LLD should not be overlooked. They found that the most likely gait deviations were also in the sagittal planes and consisted of rearfoot and ankle dorsiflexion and inversion, knee flexion and adduction, hip adduction and flexion, and pelvic trendelenburg.
The sagittal, or right/left plane, and frontal, or front/back, plane involvement is consistent with the differential diagnosis of sacroiliac dysfunction, low back pain, and pronation. Really, one could justify why a LLD could contribute to pain and dysfunction in most of the lower body. It is reasonable to think that these compensational moments in gait over a long period create boney changes in the lower extremities which may contribute to low back pain.
Clinically, a leg length discrepancy can be assessed directly with a tape measure or indirectly with a shoe lift. Badii (2014) found a higher interrater reliability with the indirect method of a shoe lift as opposed to measuring with a tape measure.
Rannisto et al (2019) looked at leg length discrepancy among meat cutters with low back pain. All participants had been working for 10 years and were greater than 35 years old. Participants needed to have a LLD of 5mm (5mm is 0.5 cm) or more and complain of low back pain of >2/10 on visual analog scale (VAS). They were all given insoles and randomized into 2 groups; the intervention group were given lifts to correct the LLD about 70%; for example a 10mm LLD was corrected to 3 mm. The LLD was measured with a laser ultrasound technique. Participants were followed for 12 months. The intervention group had improvement in low back pain intensity, sciatica intensity, and took less sick time. Possibly the most amazing part is that for those that wore the heel lift at work the compliance was good.
Leg length discrepancy can often be an underlying component contributing to complaints of pain and dysfunction. It may have more of an effect on the populations who stand or walk for most of their work, and I wonder as more people transition to standing desks if we will see more people come into the clinic with a previously undiagnosed LLD.
My biggest clinical pearls from this research is that:
- Heel lifts can be used to diagnose and then for treatment (yay! One less step of getting the tape measure out)
- The heel lift does not have to be perfect. Clinically, I will try a lift and have the person walk, and then we can make a team decision if this lift is enough and feels better
- The gait compensations are consistently adduction and internal rotation throughout the lower body chain. I will continue to work on the opposing muscle groups; lateral rotators, hip extensors and abductors.
Leg Length Discrepancy can be evaluated using various assessments. To learn orthopedic evaluative techniques for patients, consider joining Lila Abbate in her course Advanced Orthopedic Assessment for the Pelvic Health Therapist.
Maziar Badii, A Nicole Wade, David R Collins, Savvakis Nicolaou, B Jacek Kobza, Jacek A Kopec, Comparison of lifts versus tape measure in determining leg length discrepancy; Journal of Rheumatology 2014, 41 (8): 1689-94
Renan A. Resende, Renata N. Kirkwood, Kevin J. Deluzio, Silvia Cabral, Sérgio T. Fonseca. "Biomechanical strategies implemented to compensate for mild leg length discrepancy during gait" Gait & Posture, Volume 46, 2016; 147-153, https://doi.org/10.1016/j.gaitpost.2016.03.012
Sam Khamis, Eli Carmeli, Relationship and significance of gait deviations associated with limb length discrepancy: A systematic review, Gait & Posture, Volume 57, 2017, 115-123, https://doi.org/10.1016/j.gaitpost.2017.05.028
Burke Gurney, Leg length discrepancy, Gait & Posture, Volume 15, Issue 2, 2002, Pages 195-206, https://doi.org/10.1016/S0966-6362(01)00148-5.
Satu Rannisto, Annaleena Okuloff, Jukka Uitti, et al. Correction of leg-length discrepancy among meat cutters with low back pain: a randomized controlled trial. BMC Musculoskeletal Disorders. 2019;(1):1. doi:10.1186/s12891-019-2478-3.
Everyday we as pelvic rehab providers get to help patients achieve their goals by meeting them where they are and guiding them along.
A couple of months ago I had a new patient come in to see me who was seven months status post c-section delivery of her first child. She was referred to physical therapy because she could not tolerate anything touching her lower abdomen and she was also unsure of how to start exercising again including returning to her yoga practice. I remember reading her referral and thinking that this should be a simple evaluation and treatment session. What actually happened was a little different.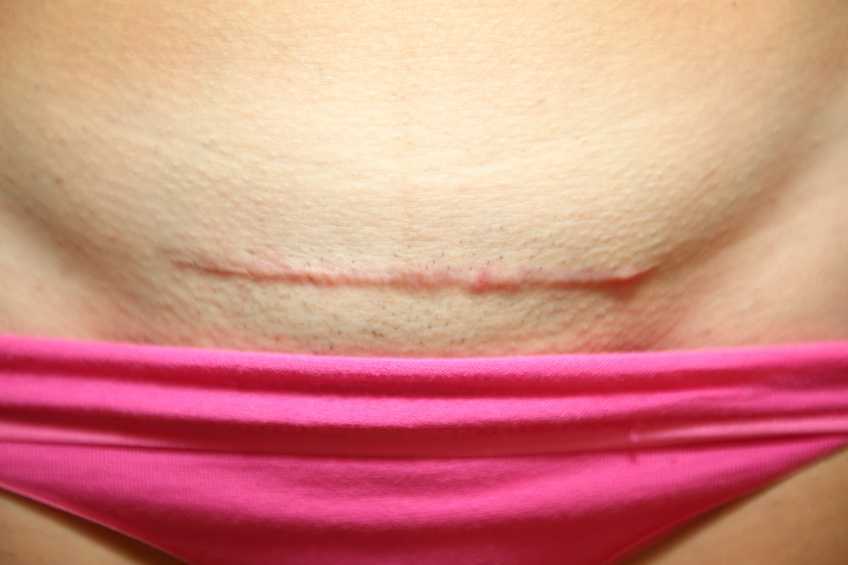
Her delivery hadn’t gone the way she planned, and she was not comfortable discussing it at our first session. This patient had not looked at or touched her c-section incision besides drying it off after her shower for the seven months since delivery. Her physician had made a referral to PT and to a counselor within three months of delivery to help support the patients’ recovery. The patient had not followed through with the PT referral until she had significant encouragement from her counselor and physician.
Initially the patient declined any observation or palpation of her abdomen so at our first session we focused on thoracic range of motion, general posture, and encouraged her to start touching her abdomen through her clothes, even if avoiding direct touch to the incisional region. The patient was agreeable with this starting point. At the second session the patient was willing to have me look at her abdomen and touch the abdomen but she declined direct palpation of the scar region. With simple observation I could see a scar that was closed and healing but also that was pulled inferior towards her pubic bone. She was not comfortable laying flat on the treatment table and had to be supported in a semi-recline throughout the session. She also described buzzing symptoms at the scar region when she reached her arms overhead.
We started some gentle desensitization techniques as would be used with a person that had Complex Regional Pain Syndrome (CRPS) after an injury. I focused those treatments to the abdominal region but avoided the scar region. We focused her home program on breathing into her abdomen allowing some stretch and expansion of the abdominal region. Her home program also included laying flat for five minutes per day. I asked her to notice any general tension throughout her body during the day and attempt to change it and release it if able.
By the fourth session we where able to begin direct palpation and manual therapy techniques to the c-section scar and the whole abdominal region. The patient was apprehensive but agreed to proceeding with utilizing techniques as described by Wasserman et al2018 including superficial skin rolling, direct scar mobilization and general petrissage/effleurage of the abdomen and lumbothoracic region.
Over the next five sessions the patient was able to start wearing undergarments and pants that touched her lower abdomen. She was able to perform her own self massage to the region and began an exercise program including prone press ups, progressive generalized trunk strengthening, and return to her prior-to-pregnancy yoga practice.
Drawing on the techniques we learn from multiple sources, applying them to the lumbopelvic region, and helping our patients wherever the client is in their journey to wellness, is what inspires me to keep learning.
Techniques like this are taught in my 2-day Manual Therapy Techniques for the Pelvic Rehab Therapist course. I specifically wrote this course so that pelvic rehab therapists that are looking for more techniques and/or more confidence in their palpation skills would have a weekend to hone those skills. We spend time learning anatomy, learning palpation skills, manual techniques, problem solving home programs and discussing cases. Check out Manual Therapy Techniques for the Pelvic Rehab Therapist - Raleigh, NC - June 22-23, 2019 for more information and I hope to see you there.
Wasserman, J. B., Abraham, K., Massery, M., Chu, J., Farrow, A., & Marcoux, B. C. (2018). Soft Tissue Mobilization Techniques Are Effective in Treating Chronic Pain Following Cesarean Section: A Multicenter Randomized Clinical Trial. Journal of Women’s Health Physical Therapy, 42(3), 111-119.
In the United States, estimated direct medical costs for outpatient visits for chronic pelvic pain (CPP) is more than $2.8 billion per year.1 In a 2017 study in the Clinical Journal of Pain by Sanses et al, a detailed musculoskeletal exam of clients with CPP can assist both physicians as well as physical therapists in differential diagnosis and appropriate referrals for this population.
Evaluating a client with pelvic pain requires a skill set that includes direct pelvic floor as well as musculoskeletal test item clusters. The prioritization of which depends upon many factors including clinician discipline, experience, specialty vs. general setting, as well as client history, presentation and goals. In addition to the direct pelvic floor assessment, there are additional key musculoskeletal screening tests that are an essential part of a pelvic pain assessment. New this year, my course Finding the Driver in Pelvic Pain will incorporate the use of Real Time Ultrasound in neuromuscular assessment and re-education of the pelvic floor and abdominal wall during the Sunday morning lab session.
Peery et al (2012) noted that abdominal pain was one of the most common presenting reasons for an outpatient physician visit in the United States. Abdominal pain is one of the many complaints that our clients may report requiring differential diagnosis including urogynecologic, colorectal, musculoskeletal, visceral or neurogenic causes. Lower abdominal quadrant pain may denote serious emergent pathology. Clinical findings, physical exam and client symptoms in addition to smart differential diagnosis must be used to determine if the abdominal pain is musculoskeletal in nature. Direct access requires physical therapists to perform a skilled initial screening for abdominal pain in order to determine if it is abdominal wall versus a visceral origin. Physicians are fluent in ruling out emergent pathology but may not be familiar with musculoskeletal tests for non-emergent pathology. Assessment of bowel and bladder function and habits are essential to perform. This blog specifically addresses three physical exam tests that can be performed as part of abdominal wall pain screening. According to Cartwright et al, the location of the abdominal pain should drive the evaluation.
Carnett’s test is a simple clinical test that assesses abdominal pain response when a client tenses their abdominal muscles. A positive Carnett’s sign denotes the origin of symptoms within the abdominal wall with a negative tests suggesting intra-abdominal pathology. The test is performed in supine, the clinician gently palpating the area of abdominal pain and has the client lift their head and shoulders off the table. Conditions such as myofascial trigger points, scar and muscular pain would be flared with palpation of the contractile tissue with activation of the abdominal wall muscles. If the pain is due to visceral origin, appendicitis for example, the pain would remain unchanged with palpation with head lift. Although some perform Carnett’s test by lifting both legs off the table, this method may cause unnecessary pain in clients with poor lumbopelvic control. (Figure 1) The head and shoulder lift option is felt to be comparable method of performing Carnett’s test.
Blumberg’s sign is most commonly used to rule in appendicitis, peritonitis or a visceral driver of right lower quadrant pain. The test is performed by the clinician applying deep pressure over McBurney’s point (Figure 2) with an abrupt and rapid release of pressure. Although there are anatomical variations in appendix location, pain reproduction is consistent with a positive test and immediate referral to the ER is indicated.
Thoracic dysfunction, including disc herniation, can result in abdominal pain.2 In thoracic discogenic driven abdominal pain, symptoms would likely be exacerbated by coughing, sneezing, spinal flexion and activities that would increase spinal loading. A simple screening for this is seated thoracic traction. If the client reports reduction or resolution of symptoms with traction, further musculoskeletal tests including regional movement and PIVM testing could be implemented to rule in or rule out need for diagnostic imaging.

In the Herman Wallace course “Finding the Driver in Pelvic Pain” participants learn a comprehensive musculoskeletal screen including abdominal, neural mobility and conductivity, pelvic ring, pelvic floor and biomechanical contributing factors to pelvic pain. Evidence based test item clusters are defined, along with their diagnostic accuracy, for all associated systems in order to outline a comprehensive screen for pelvic pain clients. To learn more about musculoskeletal screening for pelvic pain, check out faculty member Elizabeth Hampton PT, DPT, WCS, PRPC, BCB-PMD’s course Finding the Driver of Pelvic Pain, which is next offered Jun 28, 2019 - Jun 30, 2019 in Columbus, Ohio. We are fortunate to have Dick Poore, President of The Prometheus Group present on Sunday June 30th for technical support for the Real Time Ultrasound portion of the course.
1. Sanses et al. "The Pelvis and Beyond: Musculoskeletal Tender Points in Women with Chronic Pelvic Pain". Clin. J. Pain. 2016 Aug. doi: 10.1097/AJP.0000000000000307
2. Papadakos et al. "Thoracic Disc Prolapse Presenting with Abdominal Pain: Case Report and Review of the Literature". Ann. R. Coll. Surg. Engl. 20019 Jul. doi: 10.1308/147870809X401038
“My butt hurts.” This is such a common subjective complaint in my practice as a manual therapist, and many patients insist it must be a muscle problem or jump to the conclusion it must be “sciatica.” I often tell patients if they did not get shot or bit directly in the buttocks, the pain is most likely referred from nerves that originate in the spine. Although blunt trauma to the buttocks can certainly be the culprit for pain in the gluteal region, a basic understanding of the neural contribution is essential for providing appropriate treatment and a sensible explanation for patients.
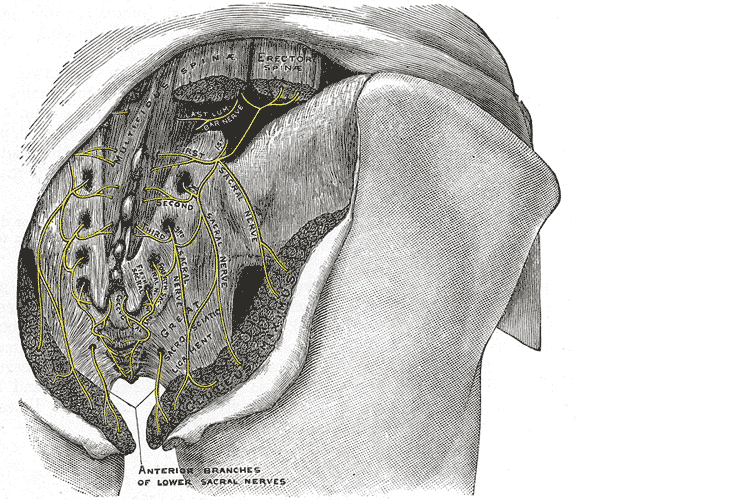 A publication by Lung and Lui (2018) describes the superior gluteal nerve. It comes from the dorsal (posterior) divisions of the L4, L5, and S1 nerve roots of the sacral plexus and innervates the gluteus medius, gluteus minimus, and tensor fasciae latae muscles. When this nerve is damaged or compressed, a Trendelenburg gait results because of paralysis of the gluteus medius muscle. The gluteus minimus and tensor fascia latae muscles are also innervated by the superior gluteal nerve and form the “abductor mechanism” together with the gluteus medius to stabilize the pelvis in midstance as the opposite leg is in swing phase. The superior gluteal nerve courses with the inferior gluteal nerve, sciatic nerve, and coccygeal plexus, but it is the only nerve to exit the greater sciatic foramen above the piriformis muscle.
A publication by Lung and Lui (2018) describes the superior gluteal nerve. It comes from the dorsal (posterior) divisions of the L4, L5, and S1 nerve roots of the sacral plexus and innervates the gluteus medius, gluteus minimus, and tensor fasciae latae muscles. When this nerve is damaged or compressed, a Trendelenburg gait results because of paralysis of the gluteus medius muscle. The gluteus minimus and tensor fascia latae muscles are also innervated by the superior gluteal nerve and form the “abductor mechanism” together with the gluteus medius to stabilize the pelvis in midstance as the opposite leg is in swing phase. The superior gluteal nerve courses with the inferior gluteal nerve, sciatic nerve, and coccygeal plexus, but it is the only nerve to exit the greater sciatic foramen above the piriformis muscle.
Iwananga et al., (2018) presents a very recent article regarding the innervation of the piriformis muscle, which has been suspected to be the superior gluteal nerve, by dissecting each side from ten cadavers. Often the piriformis muscle can be compromised through total hip replacements with a posterior approach, hip injuries, or chronic pain disorders. This particular study verifies there is no singular nerve that innervates the piriformis muscle, and the most common innervation sources are the superior gluteal nerve (70% of the time) and the ventral rami of S1 (85% of the time) and S2 (70% of the time). The inferior gluteal nerve and the L5 ventral ramus were each found to be part of the innervation only 5% of the time.
Wang et al., (2018) focused on what causes gluteal pain with lumbar disc herniation, particularly at L4-5, L5-S1. They emphasize the important factor that dorsal nerve roots have sensory fibers and ventral roots contain motor neurons, and spinal nerves are mixed nerves, since they have ventral and dorsal roots. They discuss other contributing nerves, but continuing our focus on the superior gluteal nerve, it stems from L4-S1 ventral rami and not only allows movement of gluteus medius, gluteus minimus, and gluteus maximus, it also provides sensation to the area. This nerve can certainly produce pain in the gluteal region when irritated. In lumbar disc herniation of L4-5 or L5-S1, the ventral rami of L5 or S1 can be comprised or irritated at the level of the nerve root and provoke gluteal pain because they mediate sensation in that area.
Once the superior gluteal nerve (or any sacral nerve) is implicated as the root of pain, should we just shrug our shoulders and send them to pain management? I strongly suggest we learn how to address the issue in therapy using our hands with manual techniques and appropriate exercises. The Sacral Nerve Manual Assessment and Treatment course should be a priority on your bucket list of continuing education to help alleviate any further pain in the butt.
Lung K, Lui F. Anatomy, Abdomen and Pelvis, Superior Gluteal Nerve. [Updated 2018 Dec 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535408/
Iwanaga J, Eid S, Simonds E, Schumacher M, Loukas M, Tubbs RS. (2018). The Majority of Piriformis Muscles are Innervated by the Superior Gluteal Nerve. Clinical Anatomy. doi: 10.1002/ca.23311. [Epub ahead of print]
Wang, Y., Yang, J., Yan, Y., Zhang, L., Guo, C., Peng, Z., & Kong, Q. (2018). Possible pathogenic mechanism of gluteal pain in lumbar disc hernia. BMC musculoskeletal disorders, 19(1), 214. doi:10.1186/s12891-018-2147-y
Tibial nerve stimulation has been shown in the literature to be effective for individuals experiencing idiopathic overactive bladder in randomized controlled trials. A systematic review was performed by Schneider, M.P. et al. in 2015 looking at safety and efficacy of its use in neurogenic lower urinary tract dysfunction. Many variables were examined in this review, which included 16 studies after exclusion. The review looked at:
- Acute stimulation (used during urodynamic assessment only)
- Chronic stimulation (6-12 weeks of daily-weekly use)
- Percutaneous or transcutaneous (frequencies, pulse widths, perception thresholds, durations)
- Urodynamic parameter changes baseline to post treatment
- Post void residual changes
- Bladder diary variables
- Patient adherence to tibial nerve stimulation
- Any adverse events
The exact mechanism of these types of neuromodulation stimulation procedures remains unclear, however it does appear to play a role in neuroplastic reorganization of cortical networks via peripheral afferents. No specific literature is currently available for the mechanism on action related to neurogenic lower urinary tract dysfunction. Different applications of neuromodulation however have been studied in the neurogenic populations.
One of the randomized controlled trials they report on included 13 people with Parkinson disease. The researchers looked at a comparison between the use of transcutaneous tibial nerve stimulation (n = 8) and sham transcutaneous tibial nerve stimulation (n=5). Transcutaneous tibial nerve stimulation (TTNS) or sham stimulation was delivered to the people with Parkinson disease 2x/week for 5 weeks, 30-minute sessions (10 total sessions). Unilateral electrode placement was utilized, first electrode applied below the left medial malleolus and second electrode 5 cm cephalad. Confirmation of placement was obtained with left great toe plantar flexion. It is important to note the use of the stimulation intensity is reduced to below the motor threshold during the active treatment to direct the stimulation via peripheral afferents.
Urodynamic testing was performed at baseline and post treatment and revealed statistically significant differences with greater volumes at strong desire and urgency in the TTNS group. Additionally, the TTNS group experienced a 50% reduction in nocturia whereas in the sham group nocturia frequency remained the same. A three-day bladder diary completed by each of the groups also revealed significant positive changes in frequency, urgency, urge urinary incontinence and hesitancy only in the TTNS group.
Conservative management of neurogenic bladder in populations such as Parkinson disease is very important. These individuals experience lower quality of life ratings related to lower urinary tract dysfunction, higher risk of falling with needs to rush to the bathroom, their caregivers experience a higher level stress and burden of care, and tolerance to anticholinergic medications is very poor with multiple unwanted side effects that compound and worsen other symptoms that might be present from the disease process.
Please join us for Neurologic Conditions and Pelvic Floor Rehab to learn how you can help your patients using this modality as one option. Participate in a lab session to learn electrode placement and other parameters to achieve best clinical results for your patients.
1. Perissinotto, M. C., D'Ancona, C. A. L., Lucio, A., Campos, R. M., & Abreu, A. (2015). Transcutaneous tibial nerve stimulation in the treatment of lower urinary tract symptoms and its impact on health-related quality of life in patients with Parkinson disease: a randomized controlled trial. Journal of Wound Ostomy & Continence Nursing, 42(1), 94-99.
2. Schneider, M. P., Gross, T., Bachmann, L. M., Blok, B. F., Castro-Diaz, D., Del Popolo, G., ... & Kessler, T. M. (2015). Tibial nerve stimulation for treating neurogenic lower urinary tract dysfunction: a systematic review. European urology, 68(5), 859-867.
The following is the first in a series on self-care and preventing practitioner burnout from faculty member Jennafer Vande Vegte, MSPT, BCB-PMD, PRPC. Jennafer is the co-author and co-instructor of the Boundaries, Self-Care, and Meditation course along with Nari Clemons, PT, PRPC.
Part 1: Boundaries
“I just want you to fix me.” How many times have we heard this statement from our patients? And how do we respond? In my former life as a “rescuer” this statement would be a personal challenge. I wanted to be the fixer, find the solution and identify the thing that no one else had seen yet. Then, if I am being completely honest, bask in the glory of being the “miracle worker” and “never giving up” on my patient.
 If you recognize that this attitude was going to run me into some problems, kudos to you. If you are thinking, “well of course, isn’t that your job as a pelvic floor physical therapist?” Please read on.
If you recognize that this attitude was going to run me into some problems, kudos to you. If you are thinking, “well of course, isn’t that your job as a pelvic floor physical therapist?” Please read on.
On my very first job performance review, when it came time to discuss my problem areas my supervisor relayed I was “too nice” and cited some examples: giving a patient a ride home after therapy (it was raining and she would have had to wait for the bus), coming in on Saturdays to care for patients (he was sick and couldn’t make it in during the week but was making really good progress). You get the picture. At the time, I didn’t understand how this could be something I needed to work on. I was going above and beyond and I got so much satisfaction from taking care of others!
Fast forward 10 years and add to my life a husband, two daughters, a teaching job, part time homeschooling, and writing course material. I was an emotional mess. Anxiety was my permanent state of mind. I gave my best to my patients while my family got my meager emotional leftovers. Something had to change and luckily it did. I got help and learned exactly what boundaries are and how to develop as well as enforce them.
There are several resources that discuss professional boundaries in health care, like this from Nursing Made Incredibly Easy. In this particular article, health care professionals are exhorted to stay in the “zone of helpfulness” and avoid becoming under involved or over involved with patients. Health care professionals are also urged to examine their own motivation. Am I using my relationship with my patient to fulfill my own needs? Am I over involved so that I can justify my own worth?
Here are some warning signs that you are straying away from healthy boundaries with patients and becoming over involved:
- Discussing your intimate or personal issues with a patient
- Spending more time with a patient than scheduled or seeing a patient outside of work
- Taking a patient's side when there's a disagreement between the patient and his or her close relations
- Believing that you are the only health care member that can help or understand a patient
For some people, certain patients who push professional boundaries will cause the therapist to feel threatened and under activity is the result. This might result in talking badly about the patient to other staff, distancing ourselves, showing disinterest in their case, or failing to utilize best care practices for the patient.
Per Remshard 2012, “When you begin to feel a bit detached, stand back and evaluate your interactions. If you sense that boundaries are becoming blurred in any patient care situation, seek guidance from your supervisor. A sentinel question to ask is: ‘Will this intervention benefit the patient or does it satisfy some need in me?’”
Healthy professional boundaries are imperative for us and for our patients. Boundaries also help prevent burnout. Remshard delineates what healthy boundaries look like:
- Treat all patients, at all times, with dignity and respect.
- Inspire confidence in all patients by speaking, acting, and dressing professionally.
- Through your example, motivate those you work with to talk about and treat patients and their families respectfully.
- Be fair and consistent with each patient to inspire trust, amplify your professionalism, and enhance your credibility.
If you struggle with professional and personal boundaries, you are not alone and you can get support. Consider talking with your supervisor, a counselor, reading a good book on the subject or taking Boundaries, Self-Care, and Meditation, a course offering through Herman and Wallace that was designed to help pelvic health professionals stay healthy and inspired while equipping therapists with new tools to share with their patients.
We hope you will join us for Boundaries, Self-Care, and Meditation this November 9-11, 2019 in San Diego, CA.
Look forward to my next blog where The Rescuer (me) needs Rescuing and learn about the Drama Triangle.
Remshardt, Mary Ann EdD, MSN, RN "Do you know your professional boundaries?" Nursing Made Incredibly Easy!: January/February 2012 - Volume 10 - Issue 1 - p 5–6 doi: 10.1097/01.NME.0000406039.61410.a5
Diagnosing sacroiliac joint (SIJ) dysfunction can be tricky. Therapists need to rule out lumbar spine and the hip, and sometimes there is more than one area causing pain and limiting functional mobility. Typically, ruling in SIJ dysfunction is done by pain provocation tests and load transfer tests. Once the SIJ has been ruled in, then therapists can use a variety of treatments. Often those treatments include therapeutic exercise, joint manipulation, and Kinesio tape. But which intervention is the most effective?
A recent study looked at three physical therapy interventions for treatment for SIJ (sacroiliac joint) dysfunction and assessed which was the most effective (Al-Subahi, M 2017). The authors did a systematic review of the literature. The articles were from 2004-2014, written in English, with male and female participants. This review included a variety of experiment types from randomized control trials to case studies. Of the 1114 studies, only 9 met the inclusion and exclusion criteria. Four of the nine studies used manipulation, three used Kinesio Tape, and the three used exercise. One study did both exercise and manipulation, and was looked at in both interventions. All categories had at least one randomized control trial.
For the manipulation intervention, all studies showed a decrease in pain and disability at follow up. The follow ranged from 3 to 4 days to 8 weeks. Disability was measured using the Oswestry Disability Index. One study did manual high velocity and low amplitude thrust manipulation to lumbar and SIJ manipulation and showed improvement with manipulation to SIJ or SIJ and lumbar. The review did not disclose the type of lumbar manipulation, but did state the SIJ manipulation was a side bend and rotation position with an inferior and lateral force to ASIS (anterior superior iliac spine). Another study did either a SIJ manual high velocity and low amplitude thrust manipulation or a mechanical force with manual assistance. One studied did manipulation and home exercises but did not record exercise interventions. The last study did the same SIJ manual high velocity and low amplitude thrust manipulation as in previous study combined with exercise. The exercises are mentioned below.
For the exercise intervention, the studies did primarily stabilization exercises that were either isometric or isotonic eccentric or concentric. Quick PT school review, in isometric exercises the muscle does not change length, while in isotonic eccentric exercises the muscle is being lengthened under load, and isotonic concentric is the muscle shortening under load. All three studies showed decrease in pain. The first study had 7 participants and combined manipulation and exercise. The exercises consisted of 12% max voluntary contraction and eccentric loading quads in supine with hips at 90 degrees, and concentrically loading hamstrings in prone. The second study was a case study and performed 8 lumbo-pelvic-femoral stabilization exercises for 8 weeks. Fun fact: this case study was written by my Therapeutic Exercise teacher in PT school who did a lot of Postural Restoration based exercises. The last study, had 22 participants and educated and provided exercises on deep abdominal and multifidus muscles and do complete these exercises during functional movements throughout the day. These participants were follow up a year later and had decreased pain compared to laser group.
For the Kinesio tape (Kinesio tape) intervention, the studies did not find that Kinesio tape was not an effective intervention, however the follow up ranged from immediately after applying tape to 4 weeks afterwards. In the first study, a randomized controlled trial with 60 participants, the Kinesio tape was applied in sitting with 25% tension of 4 strips making a star pattern over the point of maximal pain. The Kinesio tape was compared to placebo tape and showed equal improvement in pain and disability. The other two studies applied a different taping technique where the Kinesio tape was applied. One applied the tape over erector spinae and internal oblique muscles bilaterally and in the other study the Kinesio tape was applied with 25% tension over external obliques, a second strip was placed from ASIS to PSIS in side-lying, and then a third strip was placed along rectus abdominis muscle. In this same study the tape was applied for weeks (6x/week for 9 hours/day).
In summary, the authors note that all three interventions help decrease pain and disability in women and men with SIJ dysfunction. The authors suggest that manipulation may be the most effective. Kinesio tape showed no significant difference between placebo tape. Exercise was effective, but less so than manipulation.
This review has a lot of limitations. The variety of experiment types with varying degrees of evidence, small number of participants, and lack of blinding. Most studies had a limited follow up ranging from 3-4 days to 12 months. The outcome measures varied greatly. Most studies had pain scores as the outcome measure, though one study only used inclination meter of anterior pelvic tilt. Use of a consistent objective measure in addition to perceived pain and disability would have helped. Only 1 study did pain provocation tests and that study was a case study whose intervention focused on Kinesio-taping.
As physical therapists we want to provide effective evidence-based practice, and we want to provide individualized compassionate care. It is hard to make a direct line between this study’s recommendations and clinical application based on the numerous limitations. I agree with the authors that manipulation and exercise are bread and butter to physical therapists. I disagree about Kinesio tape not being an effective treatment. Is Kinesio tape going to create boney alignment changes? Likely not. Is Kinesio tape (or any other tape) going to give proprioceptive feedback and possibly help calm sensory pathways? Yes. If a patient likes being taped, and thinks it will help, then I will tape them. Even if taping is just placebo effect; it’s still an effect.
Al-Subahi, M., Alayat, M., Alshehr, M.A., et al. (2017) The effectiveness of physiotherapy interventions for sacroiliac joint dysfunction: A systematic review. J Phys. Ther. Sci. 29: 1689-1694.
Rehabilitative ultrasound imaging has been used in clinical practice for well over a decade now. It has been used for core stabilization, as well as with female incontinence patients. In recent years, transperineal ultrasound imaging has emerged as a useful tool for assessing prolapses and identifying other women’s health issues in the anterior compartment.
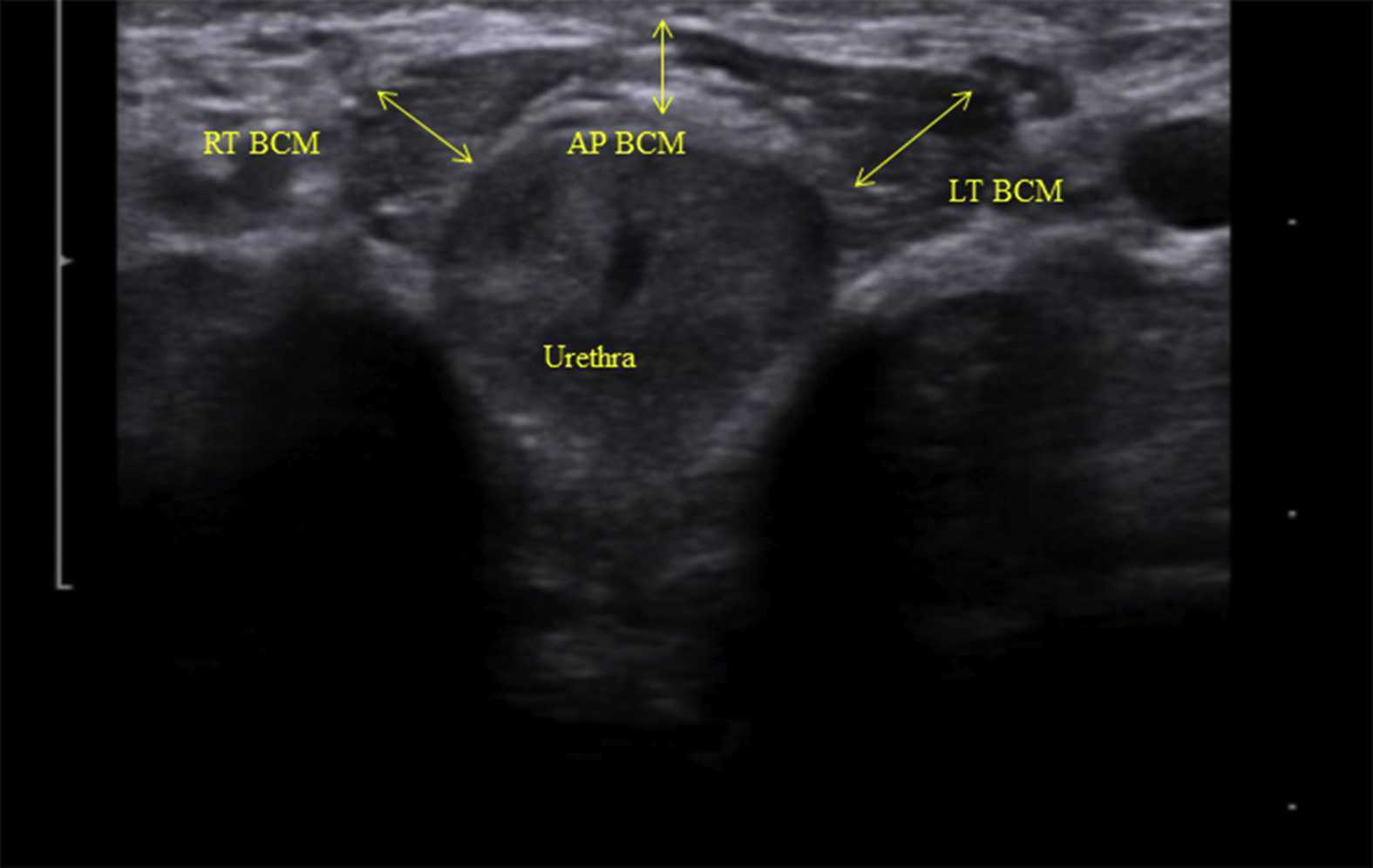 Like other things in men’s pelvic health, the use of ultrasound imaging for rehabilitation has lagged behind that in women’s pelvic health. Ryan Stafford is a researcher that is working to change that. In 2012, Stafford began looking at the normal responses to pelvic floor contractions and what is seen on ultrasound in men. He has since taken his research further to examine differences in men that present with post-prostatectomy incontinence. Stafford, van den Hoorn, Coughlin, and Hodges performed a study looking at the dynamic features of activation of specific pelvic floor muscles, and anatomical parameters of the urethra. The study included forty-two men who had undergone prostatectomy. Some of these men were incontinent and others remained continent. Transperineal ultrasound imaging was used to obtain images of the pelvic structures during a cough, and a sustained maximal contraction. The research team calculated displacements of pelvic floor landmarks with contraction, as well as anatomical features including urethral length, and resting position of the ano-rectal and urethra-vesical junctions.
Like other things in men’s pelvic health, the use of ultrasound imaging for rehabilitation has lagged behind that in women’s pelvic health. Ryan Stafford is a researcher that is working to change that. In 2012, Stafford began looking at the normal responses to pelvic floor contractions and what is seen on ultrasound in men. He has since taken his research further to examine differences in men that present with post-prostatectomy incontinence. Stafford, van den Hoorn, Coughlin, and Hodges performed a study looking at the dynamic features of activation of specific pelvic floor muscles, and anatomical parameters of the urethra. The study included forty-two men who had undergone prostatectomy. Some of these men were incontinent and others remained continent. Transperineal ultrasound imaging was used to obtain images of the pelvic structures during a cough, and a sustained maximal contraction. The research team calculated displacements of pelvic floor landmarks with contraction, as well as anatomical features including urethral length, and resting position of the ano-rectal and urethra-vesical junctions.
The data was analyzed and combinations of variables that best distinguished men with and without incontinence were reported. Several important components were identified in the study. Striated urethral sphincter activation, as well as bulbocavernosus and puborectalis muscle activation were significantly different between men with and without incontinence. When these two parameters were examined together, they were able to correctly identify 88.1% of incontinent men. They further reported that poor function of the puborectalis and bulbocavernosus could be compensated for if the man had good striated urethral sphincter function. However, the puborectalis and bulbocavernosus had less potential to compensate for poor striated urethral sphincter function. This is important for a therapist that works with post prostatectomy patients to know. This can explain part of why some men improve and do so well after a prostatectomy and others don’t, even with therapy to help. If the striated urethra sphincter is damaged and its normal responses are changed during surgery, then incontinence after prostatectomy may be more likely.
Using ultrasound imaging, the therapist can examine and see exactly where a man is deficient in response; whether it is the puborectalis, or the striated urethra sphincter. It is exciting to see this new research and see how rehabilitative ultrasound imaging can influence men’s pelvic health! Come and learn how to use ultrasound imaging for your men’s pelvic health patients as well as your women’s health and back pain patients! You will see how ultrasound imaging can change your practice and how much your patients will enjoy seeing real-time images of their contractions! Thanks to our partnership with The Prometheus Group, this course includes hands-on training on the latest in pelvic ultrasound imaging.
1. Stafford R, Ashton-Miller J, Constantinou C, et al. Novel insights into the dynamics of male pelvic floor contractions through transperineal ultrasound imaging. J. Urol. 2012; 188: 1224-30.
2. Stafford RE, van den Hoorn W, Couglin G, Hodges P. Postprostatectomy incontinence is related to pelvic floor displacements observed with trans-perineal ultrasound imaging. Neurol and Urodyn. 2018; 37:658-665.
Image credit Gupta et al. 2016 https://doi.org/10.1016/j.ajur.2016.11.002 https://www.sciencedirect.com/science/article/pii/S2214388216300881#fig2
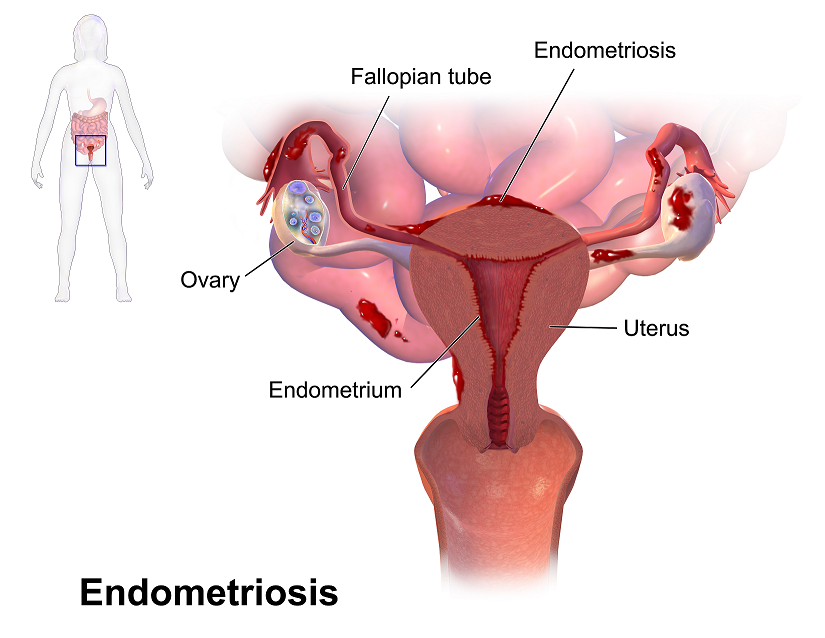
Recent data suggests that there are about 4 million American women diagnosed with endometriosis, but that 6/10 are not diagnosed. Currently, using the gold standard for diagnosis there are potentially 6 million American woman that may experience the sequelae of endometriosis without having appropriate management or understanding the cause of their symptoms.
The gold standard for endometriosis is laparoscopy either with or without histologic verification of endometrial tissue outside of the uterus. However, there is a poor correlation between disease severity and symptoms. The Agarwal et al study suggests a shift to focus on the patient rather than the lesion and that endometriosis may better be defined as “menstrual cycle dependent, chronic, inflammatory, systemic disease that commonly presents as pelvic pain”. There is often a long delay in symptom appreciation and diagnosis that can range from 4-11 years. The side effects of this delay are to the detriment of the patient; persistent symptoms and effect of quality of life, development of central sensitization, negative effects on patient-physician relationship. If this disease continues to go untreated it may affect fertility and contribute to persistent pelvic pain.
 The authors suggest a clinical diagnosis with transvaginal ultrasound for patients presenting with persistent or cyclic pelvic pain, patient history, have symptoms consistent with endometriosis, or other findings suggestive of endometriosis. The intention of using transvaginal ultrasound is to make diagnosis more accessible and limit under diagnosis. It is not intended to minimize laparoscopy as a diagnostic tool or treatment option.
The authors suggest a clinical diagnosis with transvaginal ultrasound for patients presenting with persistent or cyclic pelvic pain, patient history, have symptoms consistent with endometriosis, or other findings suggestive of endometriosis. The intention of using transvaginal ultrasound is to make diagnosis more accessible and limit under diagnosis. It is not intended to minimize laparoscopy as a diagnostic tool or treatment option.
The algorithm for a clinical diagnosis evaluates patient presentation of the following:
- Symptoms including persistent or cyclic pelvic pain, dysmenorrhea or painful menstruation cramps, deep dyspareunia or pain with deep vaginal penetration, cyclic dyschezia or straining for soft stools, cyclic dysuria or pain with urination, cyclic catamenial symptoms located in other systems such as acne or vomiting.
- Assessment of patient history including infertility, current chronic pelvic pain, or painful periods as an adolescent, previous laparoscopy with diagnosis, painful periods that are not responsive to NSAIDS, and a family history.
- Physical exam physicians assess for nodules in cul de sac, retroverted uterus, mass consistent with endometriosis, visible or obvious external endometrioma. Imaging should be ordered or performed.
- Clinical signs would consist of endometrioma with US, presence of soft markers (sliding sign) this is where the fundus of the uterus is compared to its neighboring structures and can indicate the immobility of those structures, and nodules or masses.
Of course, there are differential diagnosis for endometriosis, and those are symptoms of non-cyclical patterns of pain and bladder/bowel dysfunction that would indicate IBS, UTI, IC/PBS. A history of post-operative nerve entrapment of adhesions. Examination positive for pelvic floor spasm, severe allodynia in vulva and pelvic floor, masses such as fibroids. It is important to note that these other diagnoses can coexist with endometriosis and do not rule out possible endometriosis diagnosis.
Hopefully, diagnosing individuals earlier and possibly at a younger age would limit the disease severity and symptoms. This would allow this population to limit the possibility of central sensitization and pain persistence that can affect so much of daily life. Earlier diagnosis may affect infertility and allow this population to make informed decisions about family and career from a place of empowerment.
Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA,Singh SS, Taylor HS, "Clinical diagnosis of endometriosis: a call to action", American Journal of Obstetrics and Gynecology (2019), doi: https://doi.org/10.1016/j.ajog.2018.12.039.
The following is a guest submission from Alysson Striner, PT, DPT, PRPC. Dr. Striner became a Certified Pelvic Rehabilitation Practitioner (PRPC) in May of 2018. She specializes in pelvic rehabilitation, general outpatient orthopedics, and aquatics. She sees patients at Carondelet St Joesph’s Hospital in the Speciality Rehab Clinic located in Tucson, Arizona.
Myofascial pain from levator ani (LA) and obturator internus (OI) and connective tissues are a frequent driver of pelvic pain. As pelvic therapists, it can often be challenging to decipher whether pain is related to muscular and/or fascial restrictions. A quick review from Pelvic Floor Level 2B; overactive muscles can become functionally short (actively held in a shortened position). These pelvic floor muscles do not fully relax or contract. An analogy for this is when one lifts a grocery bag that is too heavy. One cannot lift the bag all the way or extend the arm all the way down, instead the person often uses other muscles to elevate or lower the bag. Over time both the muscle and fascial restrictions can occur when the muscle becomes structurally short (like a contracture). Structurally short muscles will appear flat or quiet on surface electromyography (SEMG). An analogy for this is when you keep your arm bent for too long, it becomes much harder to straighten out again. Signs and symptoms for muscle and fascial pain are pain to palpation, trigger points, and local or referred pain, a positive Q tip test to the lower quadrants, and common symptoms such as urinary frequency, urgency, pain, and/or dyspareunia.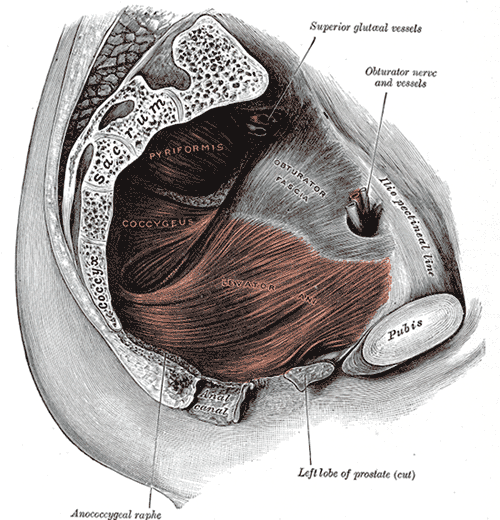
For years in the pelvic floor industry there has been notable focus on vocabulary. Encouraging all providers (researchers, MDs, and PTs) to use the same words to describe pelvic floor dysfunction allowing more efficient communication. Now that we are (hopefully) using the same words, the focus is shifting to physical assessment of pelvic floor and myofascial pain. If patients can experience the same assessment in different settings then they will likely have less fear, and the medical professionals will be able to communicate more easily.
A recent article did a systematic review of physical exam techniques for myofascial pain of pelvic floor musculature. This study completed a systematic review for the examination techniques on women for diagnosis of LA and OI myofascial pain. In the end, 55 studies with 9460 participants; 99.8% were female, that met the inclusion and exclusion criteria were assessed. The authors suggest the following as good foundation to begin; but more studies will be needed to validate and to further investigate associations between chronic pelvic pain and lower urinary tract symptoms with myofascial pain.
The recommended sequence for examining pelvic myofascial pain is:
- Educate patient on examination process. Try to ease any anxiety they may have. Obtain consent for an examination
- Ask the patient to sit in lithotomy position
- Insert a single, gloved, lubricated index finger into the vaginal introitus
- Orient to pelvic floor muscles using clock face orientation with pubic symphysis at 12 o'clock and anus at 6 o'clock
- Palpate superficial and then deep pelvic floor muscles
- Palpate the obturator internus
- Palpate each specific pelvic floor muscle and obturator internus; consider pressure algometer to standardize amount of pressure
Authors recommend bilateral palpation and documentation of trigger point location and severity with VAS. They recommend visual inspection and observation of functional movement of pelvic floor muscles.
The good news is that this is exactly how pelvic therapists are taught to assess the pelvic floor in Pelvic Floor Level 1. This is reviewed in Pelvic Floor Level 2B and changed slightly for Pelvic Floor Level 2A when the pelvic floor muscles are assessed rectally. Ramona Horton also teaches a series on fascial palpation, beginning with Mobilization of the Myofascial Layer: Pelvis and Lower Extremity. I agree that palpation should be completed bilaterally by switching hands to make assessment easier for the practitioner who may be on the side of the patient/client depending on the set up. This is an important conversation between medical providers to allow for easy communication between disciplines.
Meister, Melanie & Shivakumar, Nishkala & Sutcliffe, Siobhan & Spitznagle, Theresa & L Lowder, Jerry. (2018). Physical examination techniques for the assessment of pelvic floor myofascial pain: a systematic review. American Journal of Obstetrics and Gynecology. 219. 10.1016/j.ajog.2018.06.014